A Therapeutic Approach to Hormone Receptor Positive Metastatic Breast Cancer in a Young Female Patient
* Nicole Acosta;
Araboo L;
Kufel-Grabowska J;
-
* Nicole Acosta: Department of Electroradiology, Poznań University of Medical Sciences, Poznań 60-569, Poland.
-
Araboo L: Department of Electroradiology, Poznań University of Medical Sciences, Poznań 60-569, Poland.
-
Kufel-Grabowska J: Department of Electroradiology, Poznań University of Medical Sciences, Poznań 60-569, Poland.
-
Jan 10, 2022 |
-
Volume: 3 |
-
Issue: 1 |
-
Views: 842 |
-
Downloads: 771
-
Download PDF
Abstract
Hormone receptor-positive breast cancer is the most common subtype in women. Endocrine therapy is the recommended first-line therapeutic approach as research shows that it has the highest efficacy in managing carcinoma. Our case presents a 28-year-old female patient diagnosed with left hormone receptor-positive breast cancer that had metastasized to her bones, lymph nodes, and liver. Due to her increasingly adverse condition, chemotherapy was used as a first-line treatment, followed by endocrine therapy, and was subsequently switched back to chemotherapy. Additionally, other treatments administered were radiotherapy and bisphosphonates.
Abbreviations
BC: Breast Cancer; HR+: Hormone Receptor Positive; ER: Estrogen-Receptor; PR: Progesterone-Receptor; HR−: Hormone Receptor Negative; ET: Endocrine Therapy; ORR: Objective Response Rate; NPLD: Non-Pegylated Liposomal Doxorubicin; SREs: Skeletal-Related Events; AIs: Aromatase Inhibitors; LHRH: Luteinizing Hormone-Releasing Hormone; ABC: Advanced Breast Cancer; CBR: Clinical Benefit Rate; PFS: Progression-Free Survival.
Introduction
Breast Cancer (BC) is the most common cancer in women worldwide [1], with survival rates estimated at 80% at 15 years post-diagnosis [2]. Prevalence data indicate that BC occurs in 1% of women below 35 years [1], with research suggesting that the cancer is usually more aggressive. At an early age diagnosis, the BC is often at an advanced stage with metastasis and a considerable risk of death [3]. A study suggested that this initial presentation of advanced BC is due to the delays in diagnosing young women. Young women are typically less concerned about BC, and physicians usually do not suspect the disease immediately at this age. Furthermore, most countries do not recommend mammograms for BC screening in women < 40 years of age because there is a significantly lower sensitivity due to a higher incidence of dense breasts [4].
Statistics reveal that approximately 70% of the diagnosed BC cases are of the hormone receptor-positive (HR+) subtype, with either the estrogen-receptor (ER) and/or progesterone-receptor (PR) [5,6]. Patients can present with a tumor that is ER-positive/PR-positive or ER-positive/PR-negative or ER-negative/PR-positive. In a large cohort study, the data of 823,399 patients with BC was reviewed, and it was found that 67.2% had ER-positive/PR-positive, 12.2% had ER-positive/PR-negative, 1.6% had ER-negative/PR-positive, and 19.0% had hormone receptor-negative (HR-) tumors [7]. Additionally, this study reported that patients with single HR+ BC, such as the ER-positive/PR-negative or ER-negative/PR-positive subtypes, appeared with more aggressive clinicopathological features when compared to ER-positive/PR-positive tumors. These aggressive clinicopathological features included larger tumor size, metastasis to the lymph nodes and distant metastasis, and a higher prevalence of death related to the BC.
Endocrine Therapy (ET) is a conventional treatment for advanced HR+ BC to alleviate symptoms and uphold a good quality of life. Tamoxifen has been the ET drug of choice in treating HR+ BC and functions as a selective ER modulator, where it acts as a competitive inhibitor [2,5]. Tamoxifen binds to the ER and promotes conformational change, stopping the coactivator binding and preventing further signaling activation [2]. An extensive review of 5353 female patients treated with 20 mg–40 mg of tamoxifen daily reported an objective response rate (ORR) - defined as the proportion of patients with tumor size reduction of 34%, with 19% of these patients achieving disease stabilization [8]. Therefore, ET is used as the first-line therapy unless the patient develops endocrine resistance or a visceral crisis, where symptoms or laboratory results indicate organ dysfunction [5].
Case Presentation
This case presents a 28-year-old woman diagnosed with left de novo stage IV ductal BC, that is ER/PR-positive, with metastasis to her bones, lymph nodes, and liver. The patient had no family history of BC, nor did she have any mutations in BRCA1 and BRCA2 genes. In September 2019, the patient arrived at the hospital with a poor overall condition; she used a wheelchair due to severe back pain, dizziness, and a headache. The patient was hospitalized for five days due to the discovery of an acute renal failure. Her laboratory results indicated hypercalcemia of 4.2 mmol/L, while normal values of serum calcium range from 2 mmol/L–2.5 mmol/L. In this hospital admission, the patient was given fluids and 4 mg of zoledronic acid and was continued every 12 weeks. Additionally, a biopsy of the left breast was performed, confirming a stage IV HR+ BC diagnosis.
In October 2019, the patient received radiotherapy to her lumbar spine and right hip as these parts of her bones were most affected by the metastasis. The radiotherapy was repeated in November 2019, and she was then able to walk without the aid of a wheelchair. Due to the patient’s visceral crisis, she was started on non-pegylated liposomal doxorubicin (NPLD) from October 2019 to March 2020, where she received 60 mg/m2 every three weeks. Afterward, the patient was switched to ET, with letrozole and goserelin, as maintenance. Zoledronic acid was continued as her bone scintigraphy showed stable disease.
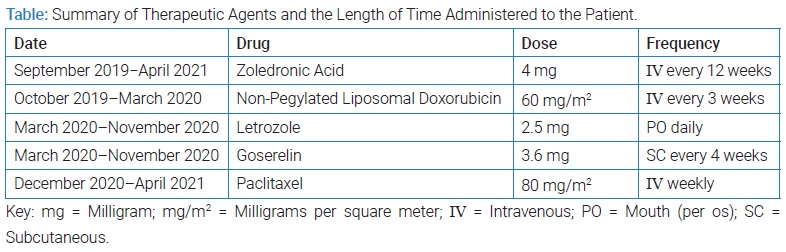
Although the patient initially showed a good response to ET, a follow-up appointment in December 2020 revealed further metastatic progression in her bones and liver. A liver biopsy was planned but never took place due to the recurrence of this visceral crisis. Subsequently, she was shifted from ET to chemotherapy, using 80 mg/m2 weekly of paclitaxel. Initially, the patient responded well to this round of chemotherapy, but this response deteriorated quickly. Eventually, liver insufficiency was the cause of the patient’s death in 2021. The (Table) shows a summary of the therapeutic agents that were administered.
Discussion
Premenopausal women are more likely to experience a poorer prognosis due to various factors such as delayed diagnosis and family history [3]. The frequency of metastases in BC declines with increasing age, with premenopausal patients 46 years and younger displaying more persistent metastases in several locations than in postmenopausal patients 47 years and older [9]. The choice of therapy for premenopausal women is critical as it determines the preservation of ovarian function, influences premature menopause, and involves psychosocial distress.
Bone metastases can lead to various Skeletal-Related Events (SREs) characterized by impaired mobility, pain, spinal cord compression, and hypercalcemia. The most common complication of a malignant disease is hypercalcemia and is frequently seen in BC patients. Serum levels greater than 3.0 mmol/L indicate severe hypercalcemia and can lead to kidney and gastrointestinal tract dysfunction. These SREs are associated with an adverse prognosis, and a multimodal therapeutic approach should be considered to palliate these symptoms. The concomitant therapy of bisphosphonates with systemic therapy is a common therapeutic approach to treating BC with bone metastases [10]. Bisphosphonates, such as pamidronate or zoledronic acid, reduce the progression of bone metastasis by inhibiting osteoclast activity and is the treatment of choice for unlocalized bone pain. Zoledronic acid is third-generation bisphosphate and effectively suppresses bone resorption and provides an analgesic effect on BC patients with bone pain and tumor-induced hypercalcemia. In a randomized trial of 228 BC patients, the patients who received 4 mg of zoledronic acid showed a 39% reduction of SREs than patients who received a placebo [11].
In combination with chemotherapy or ET, zoledronic acid significantly reduces the occurrence of SREs. ET, either alone or concomitant with other agents, is the preferred first-line treatment for patients with HR+ metastatic BC because of its high efficacy and low toxicity. For example, a combination of ET with CDK4/6 inhibitors, such as abemaciclib, has become the standard first-line treatment for patients with luminal BC [12]. In fact, a double-blind phase 3 study showed that a combination of abemaciclib and nonsteroidal aromatase inhibitors (AI) increased progression-free survival rate (PFS) to 28.2 months compared to 14.7 months with placebo and nonsteroidal AI [13]. However, due to our patient’s increasingly adverse prognosis, as evidenced by the presence of a visceral crisis, she was first treated with chemotherapy using NPLD rather than ET. Anthracyclines have a 30%–50% response rate and are the most effective drug class used in chemotherapy to treat metastatic BC. In addition, liposomal formulations of anthracyclines, such as NPLD, have been shown to reduce the risk of cardiotoxicity that is often present in anthracyclines [14]. Due to a good response to chemotherapy, the patient was then switched to ET as a form of maintenance.
The goal of hormonal therapy is to reduce estrogen levels or suppress its action with agents such as AIs (letrozole, anastrozole), luteinizing hormone-releasing hormone (LHRH) agonists (goserelin, leuprolide), or selective ER modulators (tamoxifen). Tamoxifen is a widely used anti-cancer drug, and its antiestrogenic activity on the mammary epithelium makes it a useful treatment option for premenopausal patients with ER-α positive BC [15]. Although tamoxifen is well-tolerated, potential side effects such as the increased risk of endometrial cancer and thromboembolic events are likely to occur in young patients who have been administered this drug for less than 12 months [16]. Therefore, combining an LHRH analog and AIs is another therapeutic approach for ER and/or PR-positive premenopausal women. In fact, third-generation AIs are a more favorable treatment option for premenopausal women than tamoxifen. In a double-blind, randomized phase III trial, premenopausal patients were administered neoadjuvant goserelin with either anastrozole with tamoxifen placebo or tamoxifen with an anastrozole placebo. 70.4% of patients in the anastrozole with tamoxifen placebo group experienced a complete or partial tumor benefit response compared to only 50.5% of patients who received tamoxifen with anastrozole placebo [17]. Similarly, Liu X et al. [1] reported a study of young women (≤ 35 years) with HR+ Advanced Breast Cancer (ABC) treated with goserelin and letrozole. The ORR was 25.7%, and the Clinical Benefit Rate (CBR) – defined as the proportion of patients with metastatic cancer who have complete or partial response rates and stable disease – of 65.7%. It was also found that these young patients had an average PFS of 9.6 months. For patients with disease progression, therapy was changed to chemotherapy. Comparably, a study on metastatic BC in premenopausal patients treated with letrozole and goserelin found the ORR, CBR, and PFS to be 21.1%, 71.1%, and ten months, respectively [18].
Although our patient initially responded to ET, after several months, rapid disease progression occurred. Therefore, chemotherapy was initiated again with 80 mg/m2 of paclitaxel weekly. Paclitaxel is a spindle toxin commonly used in ABC that exerts its anti-tumor effect by suspending cells in mitosis through the activation of the spindle assembly checkpoint [19]. A study completed a large multicenter phase II trial of 212 patients in which 80 mg/m2 of paclitaxel was administered weekly to women with metastatic BC. The study found weekly paclitaxel therapy to be well tolerated and reports a response rate of 21.5%, with 41% of patients experiencing disease stabilization [20]. While our patient initially responded to paclitaxel, this response was limited. Eventually, the treatment was no longer beneficial to the patient’s quality of life. Continuous rapid progression of the disease, as seen in young patients with ABC, resulted in the cessation of all treatment and eventual death.
Conclusion
Treatment selection for patients with metastatic BC warrants thorough examination based on factors such as age, menopausal status, presence of comorbidities, and prior treatment. Premenopausal women with ABC are more predisposed to an increased risk of recurrence and death than postmenopausal women. Although several therapeutic options are available, choosing the optimal therapy and sequence continues to be a challenge. An increasing number of studies are being directed towards identifying the appropriate therapeutic modality for these cases. While many of these trials show promising results, their sample size has been relatively small due to a very low prevalence of metastatic BC in young women. Additional studies are necessary to improve therapeutic efficacy.
Conflict of Interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Informed consent was obtained for this publication.
References
- Liu X, Qu H, Cao W, Wang Y, Ma Z, Li F, et al. Efficacy of combined therapy of goserelin and letrozole on very young women with advanced breast cancer as first-line endocrine therapy. Endocr J. 2013;60(6):819–28.
- Nasrazadani A, Thomas RA, Oesterreich S, Lee AV. Precision medicine in hormone receptor-positive breast cancer. Front Oncol. 2018;8:144.
- Anastasiadi Z, Lianos GD, Ignatiadou E, Harissis HV, Mitsis M. Breast cancer in young women: an overview. Updates Surg. 2017;69(3):313–317.
- Lee HB, Han W. Unique features of young age breast cancer and its management. J Breast Cancer. 2014;17(4):301–307.
- Reinert T, Barrios CH. Optimal management of hormone receptor positive metastatic breast cancer in 2016. Ther Adv Med Oncol. 2015;7(6):304–320.
- Nagaraj G, Ellis MJ, Ma CX. The natural history of hormone receptor-positive breast cancer: attempting to decipher an intriguing concept. Oncology (Williston Park). 2012;26(8):696–697, 700.
- Li Y, Yang D, Yin X, Zhang X, Huang J, Wu Y, et al. Clinico-pathological Characteristics and Breast Cancer-Specific Survival of Patients With Single Hormone Receptor-Positive Breast Cancer. JAMA Netw open. 2020;3(1):e1918160.
- Litherland S, Jackson IM. Antioestrogens in the management of hormone-dependent cancer. Cancer Treat Rev. 1988;15(3):183–94.
- Rutqvist LE, Wallgren A. Influence of age on outcome in breast carcinoma. Acta Oncol (Madr). 1983;22(4):289–294.
- Maisano R, Pergolizzi S, Cascinu S. Novel therapeutic approaches to cancer patients with bone metastasis. Crit Rev Oncol Hematol. 2001;40(3):239–250.
- Polascik TJ, Mouraviev V. Zoledronic acid in the management of metastatic bone disease. Ther Clin Risk Manag. 2008;4(1):261–268.
- Mavratzas A, Marmé F. Treatment of luminal metastatic breast cancer beyond CDK4/6 inhibition: Is there a standard of care in clinical practice? Breast Care. 2021;16(2):115–128.
- Li Z, Zou W, Zhang J, Zhang Y, Xu Q, Li S, et al. Mechanisms of CDK4/6 Inhibitor Resistance in Luminal Breast Cancer. Front Pharmacol. 2020;11:580251.
- Venturini M, Bighin C, Puglisi F, Olmeo N, Aitini E, Colucci G, et al. A multicentre Phase II study of non-pegylated liposomal doxorubicin in combination with trastuzumab and docetaxel as first-line therapy in metastatic breast cancer. Breast. 2010;19(5):333–338.
- Hu R, Hilakivi-Clarke L, Clarke R. Molecular mechanisms of tamoxifen-associated endometrial cancer (Review). Oncol Lett. 2015;9(4):1495–1501.
- Lorizio W, Wu AHB, Beattie MS, Rugo H, Tchu S, Kerlikowske K, et al. Clinical and biomarker predictors of side effects from tamoxifen. Breast Cancer Res Treat. 2012;132(3):1107–1118.
- Masuda N, Sagara Y, Kinoshita T, Iwata H, Nakamura S, Yanagita Y, et al. Neoadjuvant anastrozole versus tamoxifen in patients receiving goserelin for premenopausal breast cancer (STAGE): A double-blind, randomised phase 3 trial. Lancet Oncol. 2012;13(4):345–352.
- Park IH, Ro J, Lee KS, Kim EA, Kwon Y, Nam BH, et al. Phase II parallel group study showing comparable efficacy between premenopausal metastatic breast cancer patients treated with letrozole plus goserelin and postmenopausal patients treated with letrozole alone as first-line hormone therapy. J Clin Oncol. 2010;28(16):2705–2711.
- Neville-Webbe HL, Evans CA, Coleman RE, Holen I. Mechanisms of the synergistic interaction between the bisphosphonate zoledronic acid and the chemotherapy agent paclitaxel in breast cancer cells in vitro. Tumor Biol. 2006;27(2):92–103.
- Perez BE, Vogel CL, Irwin DH, Kirshner JJ, Patel R. Multicenter Phase II Trial of Weekly Paclitaxel in Women With Metastatic Breast Cancer. 2001;19(22):4216–4223.
Keywords
Breast cancer; Endocrine-therapy; Chemotherapy; Hormone receptor-positive
Cite this article
Acosta N, Araboo L, Kufel-Grabowska J. A Therapeutic approach to hormone receptor positive metastatic breast cancer in a young female patient. Clin Case Rep J. 2022;3(1):1–4.
Copyright
© 2022 Nicole Acosta. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-4.0).

