Apoplexy of an Adrenocorticotrophic Pituitary Macro Adenoma After Treatment of 1 Acute Myocardial Infarction
Wathik Alsalim;
Samir Al-Hakeem;
Johan Elf;
* Eva-Marie Erfurth;
-
Wathik Alsalim: Department of Endocrinology, Skåne University Hospital, Sweden.
-
Samir Al-Hakeem: Department of Internal Medicine, Landskrona Hospital, Sweden.
-
Johan Elf: Department of Medicine, Skåne University Hospital, Sweden.
-
* Eva-Marie Erfurth: Department of Endocrinology, Skåne University Hospital, Sweden.
-
Apr 13, 2020 |
-
Volume: 1 |
-
Issue: 2 |
-
Views: 2938 |
-
Downloads: 2419 |
Abstract
Background: Cushing’s disease is associated with high vascular morbidity and mortality. A pituitary adenoma apoplexy is a rare but a potentially life-threatening clinical syndrome, occurring as a result of hemorrhage or infarction in a pituitary adenoma. An important risk factor for a PA is the administration of anticoagulants. Patients with Cushing’s disease have prothrombotic changes and impairment in fibrinolysis resulting in increased risk of thromboembolism. Also an increased activation of coagulation inhibitors seems to counteract disseminated coagulation.
Case presentation: A 63 year old woman presented with hypertension, type 2 diabetes mellitus and ischemic heart disease with an ongoing myocardial infarction. The patient had clinical and laboratory findings of a pituitary adrenocorticotropic adenoma which was previously undiagnosed. The patient received anticoagulants and anti-platelet treatment for her myocardial infarction. Due to an accidental fall on her hip she suffered a massive tissue bleeding which resulted in a rapid decrease in haemoglobin followed by a pituitary apoplexy.
Conclusions: Cushing’s disease patients suffer from severely increased cardiovascular risk. In the complex pharmacological treatment of such a patient together with the spectrum of haemostatic abnormalities in CD increased awareness for a pituitary apoplexy is highly warranted.
Abbreviations
CD: Cushing’s; Disease; PA: Pituitary Apoplexy; ACTH: Adrenocorticotropic Hormone; MRI: Magnetic Resonance Imaging; VTE: Venous Thromboembolism; T2D: Type 2 Diabetes Mellitus.
Introduction
Cushing's disease (CD) is rare with 1–2 new cases per million years and often caused by an adrenocorticotropic (ACTH) producing pituitary micro adenoma. ACTH producing macro adenoma is however, unusual [1], but nevertheless occurring in 7%–20% of cases. Patients with CD suffer from high vascular morbidity and mortality [2] together with multiple adverse metabolic, musculoskeletal, and mental consequences that impact the patient’s quality of life [2]. The mortality is 4-fold compared to the general population and reported to be as high as 50% within 5 years after diagnosis [2]. This excess mortality in particularly vascular disease may persist even after successful adenoma treatment [2]. Patients with CD have an increased risk for Venous Thromboembolism (VTE), myocardial infarction and stroke [2]. As an effect of the excess of cortisol, a hypercoagulable state is reported. The mechanisms behind hyper coagulation in CD are dependent on pro-thrombotic changes in blood which includes increased levels of pro coagulant factor VIII and von Willebrand factor [3]. In addition, an impaired fibrinolytic capacity reflected in elevated concentrations of plasminogen activator inhibitor type 1 (PAI-I), TAFI and a2-antiplasmin also occurs. Ultimately these changes result in shortened activated partial thromboplastin time (APTT) and a decreased fibrinolytic potential [3]. Although of minor importance, there are also mechanisms that counteract disseminated coagulation with an increased activation of coagulation inhibitors [4,5]. Furthermore, CD patients may have other risk factors for VTE as obesity.
A pituitary adenoma apoplexy (PA) is a rare but a potentially life-threatening clinical 72 syndrome, occurring as a result of hemorrhage or infarction in a pituitary adenoma. Presenting symptoms include headache (95%), vomiting (69%), ocular paresis (78%), and reduction in visual fields (64%) or visual acuity (52%) [6]. The incidence of a PA in large series of pituitary adenomas range from 1.6%–13% and occur usually in 5% of all surgically resected pituitary adenomas [6]. Even though a PA can occur in all types of pituitary adenomas the majority (45%– 70%) was non-secreting adenomas and preferentially it occurs in macro adenomas [6]. The diagnosis of PA can be made only when a hemorrhagic infarction of a pituitary adenoma leads to the above described clinical syndrome. Magnetic resonance imaging (MRI) is the most important tool in the diagnosis of PA, being able to identify the presence of an adenoma and its hemorrhagic degeneration, and definitely superior to computed tomography, with a sensitivity ranging from 88% to 90% [7]. Many known risk factors predispose patients to symptomatic apoplexy of a pituitary adenoma, amongst which the most important are hypertension, diabetes mellitus, pituitary function dynamic tests, administration of anticoagulants, bromocriptine, estrogens and radiotherapy [8]. The exact pathogenesis underlying PA is still unknown. Suggested possible mechanism is the sub-acute excessive growth of a preexisting adenoma, which outgrows its blood supply with eventual ischemic necrosis followed by hemorrhage [8]. A few case reports have been presented with PA in an ACTH-secreting pituitary macro adenoma with a spontaneous remission [2]. We now present a fatal case of what we mean is an ACTH-producing macro adenoma, where several possible complications occurred. Further, we discuss pros and cons with anticoagulant and fibrinolytic therapy in such a difficult case.
Case Presentation
A 63 year old woman was admitted to the Internal Medicine Unit at a tertiary referral clinic with complaints of chest pain. She was diagnosed with a non ST-elevation myocardial infarction (NSTEMI) based on clinical, electrocardiography and laboratory findings i.e. plasma (P-)Troponin-T level =4994 ng/l (reference range; (ref) <15 ng/l), moreover p (plasma)-Creatinine level = 124 nmol/l (45–90), p-Haemoglobin level=120 g/l (117–153), p-INR 1.2 (INR< 1.2) and p-APPT=30 seconds (26–33) were analysed. At the age of 56 years she was diagnosed with hypercholesterolemia and hypertension and treated with ramipril 10 mg, felodipine 10 mg, hydrochlorothiazide 25 mg and metoprolol 50 mg. Some years later she was diagnosed with ischemic heart disease together with heart failure and type 2 diabetes mellitus (T2D) which was treated with metformin at a dose of 500 mg b.i.d. Furthermore, she was treated with thyroxine because of tiredness and laboratory findings of hypothyroidism, which retrospectively was confirmed as a secondary hypothyroidism. The patient was none-smoker and did not consume alcohol and she was obese (weight 94 kg and body mass index = 32 kg/m2) and had supraclavicular fat pads and abdominal obesity.
At admittance she was treated with plaque stabilization therapy i.e. acetylsalicylic acid 75 mg, ticagrelor 90 mg o.p.d and fondaparinux in a dose of 2.5 mg subcutaneously. Two weeks after admission she suffered syncope and was shortly deteriorated. An MRI of the brain revealed a large pituitary macro adenoma (20 x 20 x 30 mm) with supra-sellar dislocation of the optic chiasm (Figure 1).
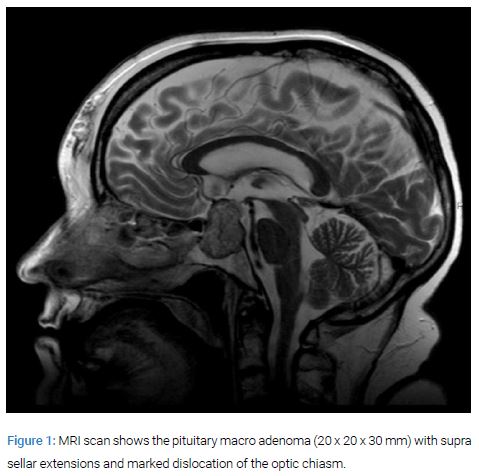
Ophthalmology consultation revealed no visual field defects and her vision was 0.13 in the right eye (due to diabetes retinopathy) and 0.7 in the left. She was without pain or other complaints when during 5 days cortisol measurements showed elevation in morning cortisol as well as in plasma afternoon levels of ACTH two days after one another (Figure 2) together with elevated urine cortisol 1310 nmol/d (ref; 38–137 nmol/day). Further, slightly elevated p-prolactin level 1035 pmol/l (ref; 65–470 pmol/l) and a suppressed serum TSH 0.04 mIE/l (ref; 0.4–3.7 mIE/l) together with normal levels of thyroid hormones with Free T3= 3.6 pmol/l and Free T4=14, (ref; 3.6–6.3 pmol/l and 12–22 pmol/l, respectively), and a normal level of serum insulin-growth factor-I of 86 µg/l 127 (ref; 43–179 µg/l) was recorded. A high dose (8 mg orally) Dexamethasone test was unsuccessful to suppress p-ACTH and p-cortisol (p-cortisol and p-ACTH; before the test; 1554 nmol/l, 52 pmol/l and after the test 1051 nmol/l, 57 pmol/l, respectively). Thus, a pituitary-dependent Cushing’s disease was suspected.
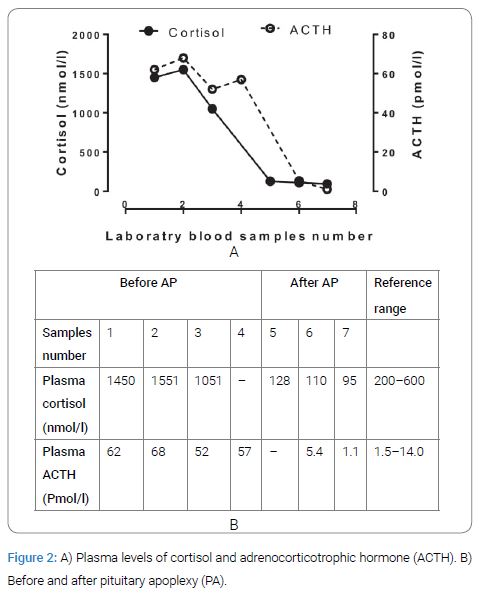
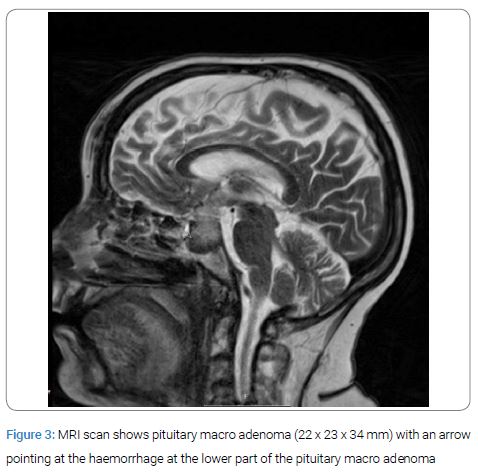
After the mentioned blood tests she fell suddenly on her hip and suffered a large haematoma on her thigh together with several haematomas all over her body. Within hours she complained of severe headache together with vomiting. Her blood pressure fell and a blood count showed a drop in haemoglobin from 106 g/l to 64 g/l (ref; 117–135 g/l) and she received blood transfusions. A computed tomography of the brain showed no sign of intracranial haemorrhage or ischemia of the brain. The day after her fall, blood samples showed a dramatic decrease in p-cortisol and in p-ACTH (Figure 2). A PA was suspected and was also confirmed by MRI (Figure 3) and she was treated with intravenous hydrocortisone. After another two weeks she was admitted to the intensive care unit because of pulmonary oedema and renal failure. She was treated with diuretics, and continuous renal replacement therapy. As she was considered too fragile for CABG she underwent coronary angiography and Percutaneous Coronary Intervention (PCI) of the left anterior descending branch of left coronary artery, left main coronary artery and left circumflex artery.
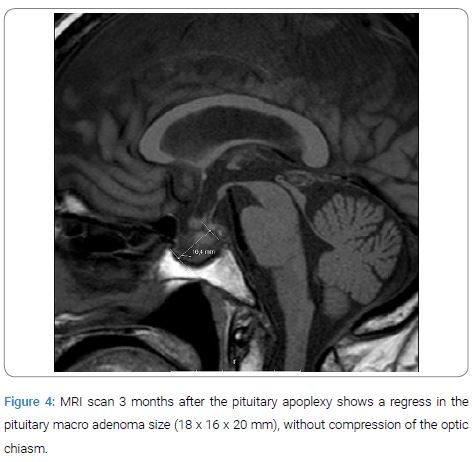
Ophthalmology consultation considered the need for surgical treatment of the macro adenoma because of diplopia as a result of left third and fourth cranial nerve palsy due to adenoma involvement of the left cavernous sinus. The visual activity after the PA confirmed a visual deterioration, in the right eye 0.06 and 0.03 in the left eye (0.13 in right eye and 0.7 left eye 3 weeks before). However, because of her severe clinical condition a pituitary adenoma operation was considered too high risk. One week later a new ophthalmological evaluation showed no further deterioration or changes regarding vision or cranial nerve status and she was discharged with oral hydrocortisone, thyroxine and cardio protective drugs. Three months after discharge a new control MRI of the pituitary sella showed a clear regression of the pituitary macro adenoma (Figure 4). Repetitive ophthalmological investigation showed improvement in her visual acuity (right eye 0.16 and left 0.2) and cranial nerve status (partial paresis in the sixth cranial nerve on left eye and in third cranial nerve on right eye).
Seven months later she died suddenly at home of a heart attack, according to paramedical report. She had had progressively shortness of breath and deterioration in her general condition before their arrival. She was declared dead after treatment with cardiopulmonary resuscitation.
Discussion
We report a patient with long-standing hypertension, T2D, obesity, hyperlipidaemia, coronary heart disease and heart failure together with central obesity and supraclavicular fat pads. Laboratory findings during a non-stressed phase showed very high levels of urinary-cortisol, p-cortisol and of p-ACTH in the afternoon (Figure 2) together with no suppression after high dose Dexametasone test. The MRI showed a macro adenoma and due to the large soft tissue haematomas with a sudden reduction in haemoglobin which affected body circulation she suffered a PA. Immediately after the PA, blood tests showed a dramatic decrease in p-cortisol from 1500 nmol/L to 110 nmol/L, and in p-ACTH from 62 pmol/l to 5.4 pmol/l respectively (Figure 2). All these symptoms and signs point in the direction of an ACTH-dependent CD in sudden remission. An acute and chronic stress might also induce augmentation of ACTH levels, however, not to such very high level of ACTH in the afternoon [9] which together with the other clinical and biochemical findings made CD diagnosis clear.
A defect in the fibrinolytic capacity in CD is reflected in elevated concentrations of Plasminogen activator inhibitor type 1 (PAI-I), TAFI and a2-antiplasmin, together with a hypercoagulable state [3] with increased levels of pro-coagulant factors i.e. 180 factor VIII and von Willebrand factor [3]. More clinically relevant is the APTT levels that may increase from drugs like ticagrelor and fondaparinux. The patient had strong indication for treatment with anticoagulants and anti-platelet treatment for preventing a recurrent myocardial infarction. The presently used treatments with acetylsalicylic acid, ticagrelor and fondaparinux are known to increase the risk of bleeding in many organs. Further, it has to be pointed out that derangements in the coagulation system caused by CD do not recover quickly after reduction in ACTH/cortisol levels instead it takes several months to restore this system [10]. Most likely the PA was a result of a traumatic massive bleeding in the soft tissue which caused a sudden drop in haemoglobin and the antithrombotic treatment probably was a predisposing factor [11]. However, the use of antiplatelet and antithrombotic therapy is considered the “gold standard” to reduce the risk of acute ischemic complications [12]. The risk for a primary bleeding in a pituitary adenoma during these circumstances is probably lower than the risk of new ischemic complications. Before the initiation of these drugs PK (INR), APT and platelet count were found to be within the normal ranges. There is also a compensatory mechanism in order to counteract disseminated coagulation e.g. protein C inhibits the procoagulant agents F VIIIa and Factor V, whereas protein S 197 supports the anticoagulant activity of activated protein in the degradation of factor Va and F VIIIa [4]. However, the net effect is still increased risk of VTE [13].
Conclusion
As illustrated in the present case CD patients suffer from severely increased cardiovascular risk. Patients with CD have pro-thrombotic changes and impairment in fibrinolysis which results in thromboses but there is also mechanism that inhibits coagulation. In the complex pharmacological treatment of such a patient increased awareness for a PA is highly warranted.
Declarations
Consent to participate: Informed consent for this case report was obtained from the patient’s first degree relative and from the head of the Hospital.
Consent for publication: Consent for publication was obtained from the patient’s first degree relative and from the head of the Hospital.
Availability of data and material: All data and materials related to this report are accessible at any time upon request.
Funding sources: The Medical Faculty, Lund University, Sweden and the Swedish Children’s Cancer Foundation.
Competing interest
Wathik Alsalim received lecture fees from Novo Nordisk and MSD, all other authors have nothing to disclose.
Author Contributions
Wathik Alsalim and Samir Al-Hakeem were involved in patient's care.
Wathik Alsalim, Johan Elf and Eva-Marie Erfurth were involved in review of literature and writing the manuscript.
All authors have approved the final manuscript.
References
- Woo YS, Isidori AM, Wat WZ, Kaltsas GA, Afshar F, Sabin I, et al. Clinical and biochemical characteristics of adrenocorticotropin secreting macroadenomas. J Clin Endocrinol Metab. 2005;90:4963–4969.
- Lambert JK, Goldberg L, Fayngold S, Kostadinov J, Post KD, Geer EB. Predictors of mortality and long-term outcomes in treated Cushing’s disease: a study of 346 patients. J Clin Endocrinol Metab. 2014;98:1022–1030.
- van der Pas R, Leebeek FW, Hofland LJ, de Herder WW, Feelders RA. Hypercoagulability in Cushing’s syndrome: prevalence, pathogenesis and treatment. Clin Endocrinol (Oxf). 2013;78:481–488.
- Dahlback B. Blood coagulation and its regulation by anticoagulant pathways: genetic pathogenesis of bleeding and thrombotic diseases. J Intern Med. 2005;257(3):209–223.
- Swiatkowska-Stodulska R, Kaniuka-Jakubowska S, Wisniewski P, Skibowska-Bielinska A, Sworczak K. The estimation of selected endogenous anticoagulation system parameters in patients with subclinical Cushing’s syndrome. Eur J Endocrinol. 2011;165(6):865–871.
- Verrees M, Arafah BM, Selman WR (2004) Pituitary tumor apoplexy: characteristics, treatment, and outcomes. Neurosurg Focus. 2004;16(4): E6.
- Onesti ST, Wisniewski T, Post KD. Clinical versus subclinical pituitary apoplexy: presentation, surgical management, and outcome in 21 patients. Neurosurgery. 1990;26(6):980–986.
- David M, Philippon J, Bernard-Weil E. Hemorrhagic forms of pituitary adenoma. Etiology and clinical aspects. Presse Med. 1969;77:1887–1889.
- Horrocks PM, Jones AF, Ratcliffe WA, Holder G, White A, Holder R, et al. Patterns of ACTH and cortisol pulsatility over twenty-four hours in normal males and females. Clin Endocrinol (Oxf). 1990;32:127–134.
- Stuijver DJ, van Zaane B, Feelders RA, Debeij J, Cannegieter SC, Hermus AR, et al. Incidence of venous thromboembolism in patients with Cushing’s syndrome: a multicenter cohort study. J Clin Endocrinol Meta. 2011;96(11):3525–3532.
- Moller-Goede DL, Brandle M, Landau K, Bernays RL, Schmid C. Pituitary apoplexy: re-evaluation of risk factors for bleeding into pituitary adenomas and impact on outcome. Eur J Endocrinol. 2011;164:37-43.
- Hamm CW, Bassand JP, Agewall S, Bax J, Boersma E, Bueno H, et al; ESC Committee for Practice Guidelines. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2011;32:2999–3054.
- Isidori AM, Minnetti M, Sbardella E, Graziadio C, Grossman AB. Mechanisms in endocrinology: The spectrum of haemostatic abnormalities in glucocorticoid excess and defect. Eur J Endocrinol. 2015;173:101–113.
Keywords
Cushing's disease; Pituitary apoplexy; Adreno corticotrophic adenoma; Anticoagulants; Myocardial infarction
Cite this article
Alsalim W, Al-Hakeem S, Elf J, Eva-Marie E. Apoplexy of an adrenocorticotrophic pituitary macro adenoma after treatment of 1 acute myocardial infarction. Clin Case Rep J. 2020;1(2):1–5.
Copyright
© 2020 Eva Marie Erfurth. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-4.0).




