Role of Adjuvant Steroid Therapy to Limit Radiation Pneumonitis in Stereotactic Body Radiation Therapy (SBRT) Treated Lung Cancer
Andrew P. Monforton;
Balkarn S. Thind;
Christopher M. Ellbogen;
Mary W. Redman;
Kelsey K. Baker;
* Shilpen A. Patel;
-
Andrew P. Monforton: Department of Medicine, University of Washington School of Medicine, United States.
-
Balkarn S. Thind: Department of Medicine, University of Washington School of Medicine, United States; Department of Medicine, University of California San Francisco Medical Center, United States.
-
Christopher M. Ellbogen: Department of Medicine, University of Washington School of Medicine, United States.
-
Mary W. Redman: Department of Medicine, Fred Hutchinson Cancer Research Center, United States.
-
Kelsey K. Baker: Department of Medicine, Fred Hutchinson Cancer Research Center, United States.
-
* Shilpen A. Patel: Department of Medicine, University of Washington School of Medicine, United States; Department of Global Health, University of Washington, United States
-
Apr 20, 2020 |
-
Volume: 1 |
-
Issue: 2 |
-
Views: 4324 |
-
Downloads: 2705 |
Abstract
Background: The median age of lung cancer diagnosis is 70 years with patients often precluded as surgical candidates due to comorbidities, tumor location, and refusal of surgery. In these cases, stereotactic body radiation therapy (SBRT) has become the treatment of choice. With SBRT, there remains a risk of radiation pneumonitis (RP) and other toxicities. Adjuvant corticosteroids could be a tool to diminish radiation toxicity. However, little data exists exploring this treatment.
Patients and Methods: This retrospective study included 121 patients diagnosed with primary lung cancer who received definitive SBRT. Patients divided into a steroid receiving group (S-group) and no steroid receiving group (NS-group). Therapy’s effect on RP and other toxicities were determined using a logistic regression model. Pairwise comparisons were performed using Fischer’s Exact Test to compare patient data, adverse events, and multiple regression. To evaluate predictors of RP Grade 2+ using a logistic regression model with p<0.05 considered as statistically significant.
Results: When comparing the overall severity of RP (Grade 1–5), no difference was found between steroid groups (P=0.06). When comparing other adverse events, the groups had a significantly different incidence of dyspnea by grade (P=0.04). The odds of developing RP Grade 2+ were not statistically different between the groups (OR 0.74; 95% CI, 0.3–1.84; P=0.52) when adjusting for other factors. In the same model, women were found to experience an increased risk of RP Grade 2+ (OR 3.78; 95% CI, 1.46–9.76; P=0.01).
Conclusions: In this study, steroid therapy was not observed to prevent RP. This study did observe an association with adjuvant steroids and dyspnea, along with identifying a patient population potentially more prone to lung injury. Further research is crucial to determine adjuvant steroid therapy’s formal role in the reduction of RP and related radiation adverse events.
Introduction
Lung cancer is the most common cancer worldwide as well as the leading cause of cancer-related death worldwide [1,2]. In the United States for 2020, the National Cancer Institute (NCI) estimates 228,820 new cases of lung cancer will be diagnosed in addition to 135,720 deaths due to this disease [3]. Unfortunately, mortality from lung cancer is the leading cause of cancer-related death in the United States (incidence of 44.7 per 100,000 deaths) [2,3]. Also, 5-year relative survival for all comers after diagnosis remains bleak at 18.1% [3]. When breaking down survival by staging, however, it becomes clear that 5-year survival vastly improves to 55.6% when diagnosed at early localized stages [3]. Since lung cancer is often asymptomatic, diagnoses are frequently made at later symptomatic stages. In fact, early-localized stage lung cancer accounts for only 16% of newly diagnosed cases [3]. Due to the grim outlook of lung cancer, it is imperative to detect and treat it in the earliest stages possible.
Historically, the basis of early-localized lung cancer treatment has been surgical resection [4–8]. Over the years, surgical methods have continued to be refined and improved. However, lung cancer often remains inoperable due to the location of the lesion, significant comorbidities, or patient refusal of surgery [3,6–8]. In these instances, other treatment modalities, such as radiation therapy (RT), is preferred. Advances in RT techniques and technology over the past two decades have considerably improved care for lung cancer patients. Stereotactic body radiation therapy (SBRT) is one such development that delivers high dose radiation to precise target volumes. This technique thereby allows maximum benefit in far fewer dosing fractions than conventional RT [7,9–12]. Trials by Timmerman et al., Grutters et al., and Grills et al., have shown that SBRT improves survival outcomes while achieving comparable local control and overall survival rates with surgical methods and conventional RT. Because of this, SBRT has become the standard of care for inoperable non-small cell lung cancer (NSCLC) [7–14].
While SBRT has drastically increased patient outcomes, adverse side effects remain a concern following any form of high dose RT. Common toxicities include pneumonitis, fatigue, dyspnea, chest wall pain, rib fracture, esophagitis, and radiation dermatitis. An especially detrimental adverse effect that occurs as a result of lung radiation exposure is radiation pneumonitis (RP), which can lead to fibrotic tissue changes [15–18]. RP is an inflammatory reaction to radiation treatment typically seen with doses of = 20 Gy, which presents with clinical symptoms of bronchitis and radiographic findings of ground-glass opacities, ill-defined patchy nodules, or consolidation and lung volume loss [15,16]. In RP, fibrotic changes often peak in severity between 6 and 12 months after SBRT treatment [17,18].
Toxicities, such as radiation pneumonitis and subsequent pulmonary fibrosis, can limit SBRT use and reduce survival rates [19–21]. In order to begin looking at the effects of SBRT and potential preventative treatments, Chiang et al. followed 41 steroid naïve patients who received spinal SBRT without steroid use [22]. They found that 68.3% of patients experienced spinal pain flairs as a main result of radiation. When rescued with 4 mg doses of dexamethasone daily, pain scores based on 13 patient pain diaries were found to significantly decrease from an average of 6/10 to less than 2/10 over the course of 9 days (P<0.0001). Based off of this promising data, Chiang et al. hypothesized prophylactic dexamethasone could be an effective strategy to reduce SBRT side effects [22].
Expanding on the findings of Chiang et al., Khan et al. sought to study the prophylactic effects of dexamethasone to prevent pain flairs for 47 patients before spinal SBRT [23]. This prospective observational study compared two cohorts receiving either 4 mg (N=24) or 8 mg (N=23) doses of dexamethasone prior to receiving SBRT therapy. The total incidence of pain flare was found to be 19%, with an insignificant statistical difference between 4 mg (25%) and 8 mg (13%) cohorts (P=0.46). When compared to the data from Chiang et al. prophylactic steroid use reduced pain flare incidence from 68% of steroid naïve patients to 19% of patients receiving preventative dexamethasone (P=<0.0001) [23]. The results by Chiang and Khan suggest a significant benefit for limiting radiation side effects through prophylactic steroid use. These studies give rise to the hypothesis that the use of preventative steroid therapy could apply to a broader scope of post-radiation symptoms and tissues.
Across the US, the use of steroids for lung cancer patients receiving radiation varies widely, given very little data exploring steroid use in any cancer form receiving SBRT. The possibility of decreasing radiation side effects with the addition of steroid regimens could present a simple method for physicians to improve patient care and quality of life. The goals of this study are: 1) Observe the incidence and severity of RP when adjuvant steroid regimens are implemented; 2) Explore possible predictors for developing RP which could include performance status, age, sex, histology, radiation dose, and staging; and 3) Inspect the incidence and severity of related radiation toxicities (cough, dyspnea, chest wall pain, fatigue, nausea, radiation dermatitis, esophagitis, and rib fracture) in steroid and non-steroid groups.
Methods
Eligibility: After Institutional Review Board approval, patient data derived from electronic medical records, radiation treatment databases, pharmacy records, radiology reports, and pathology archives from the University of Washington Medical Center (UWMC) and the Seattle Cancer Care Alliance (SCCA) in Seattle, Washington, USA. Patient inclusion criteria were defined as adults, 18–100 years of age, receiving definitive SBRT who presented with radiologic and pathologic evident inoperable primary lung cancer Stage Ia–IV (based on the AJCC Cancer Staging Manual 7th Edition) [24]. Patients were excluded for chronic steroid use prior to radiation, former conventional RT lung exposure, palliative SBRT treatment, and failure to complete prescribed radiation treatment. Patients included in this study did not have previous surgical lung cancer intervention in order to represent better the prototypical population receiving SBRT.
Data Collection: This retrospective study included 121 patients from January 2010 through June 2016 who were observed from the time of SBRT treatment through 6 to 12 months post-therapy. Data extracted from UWMC and SCCA patient records for the purpose of analysis for this study included the following information: demographics, ECOG performance status score, primary lung cancer diagnosis, cancer staging, tumor location (central or peripheral lung), volumetric tumor measurements (GTV, PTV, V20), total received SBRT dose, graded post-radiation side effects, steroid use, and histology. Graded severity of side effects were determined with the aid of Common Terminology Criteria for Adverse Events (CTCAE) version 4.02 from the U.S. Department of Health and Human Services. Graded side effects included pneumonitis, cough, dyspnea, chest wall pain, fatigue, nausea, radiation dermatitis, esophagitis, and rib fracture. Confirmation of RP was made using radiographic evidence on chest CT of ground-glass opacities, ill-defined patchy nodules, or consolidation and lung volume loss consistent with radiation lung changes. To determine if patients received dexamethasone regimens prior to SBRT, one or more of the following criteria had to be satisfied within patient records: scanned written dexamethasone prescriptions, mention of dexamethasone pre-treatment inpatient records, and/or pharmacy record of filled prescription.
Treatment Groups: As no previous data exist to recommend specific dexamethasone dosing prior to radiation treatments, all patients received a standard regimen of dexamethasone 4 mg orally 30 minutes prior to each fraction of radiation treatment. This regimen was not related to sex, age, race, body weight, type, or stage in order to provide a basis for starting steroid regimens. A total of 121 patients were divided into two main groups: steroid group (S-group, n=54) and non-steroid group (NS-group, n=67). Given steroid use variation between physicians, the relative rarity of RP and lack of previous studies, all patients eligible for this study were included to maximize patient population representation. Each received SBRT doses between 40–54 Gy over 3–5 fractions. Most commonly, patients received SBRT dose of = 50 Gy (83%). All patients completed prescribed SBRT without significant delays between fractions.
Statistical Analysis: The primary point of interest of this study was to compare steroid use in S-group versus NS-group and the incidence and severity of RP. Secondary points of interest included observing predictive qualities for developing RP (age, sex, ECOG performance status, staging, radiation dose, and histology) and adjuvant steroid therapy’s effect on the incidence of related radiation side effects (cough, dyspnea, chest wall pain, fatigue, nausea, radiation dermatitis, esophagitis, and rib fracture). Evaluations of adjuvant steroid regimens and predictors of RP in S-group versus NS-group were determined using a multivariate logistic regression model. Pairwise comparisons were performed using Fischer’s Exact Test to compare patient data, adverse events, and multiple regression. In order to evaluate predictors of RP Grade 2+, a logistic regression model was implemented with p<0.05 considered as statistically significant.
Results
Patient Characteristics: In our cohort, 121 patients met inclusion criteria, including 59 men (49%) and 62 women (51%) with ages ranged from 47 to 98 years. In the S-group, the range of age and sex were 50–98 years and 24 males/30 females, respectively. In the NS-group, the range of age and sex were 47–88 years and 35 males/32 females, respectively. The majority of patients were Caucasian (n=91, 75%) along with Asian/Pacific Islander (n=11, 9%), Black (n=10, 8%), and Native American (n=3, 2%). In the S-group, the races represented included 43 Caucasian, 3 Black, 7 Asian/Pacific Islander, and 1 Native American. In the NS-group, the races represented included 48 Caucasian, 7 Black, 4 Asian/Pacific Islander, 2 Native American, and 6 Other. Patient performance status was assessed with the Eastern Cooperative Oncology Group (ECOG) [25]. ECOG scores of 0 and 1 (n=85, 70%) were most often encountered. No patients had ECOG scores greater than three at the time of treatment. Ninety-one patients (75%) had Stage 1 lung cancer at the time of SBRT treatment. The location of lung masses was designated central or peripheral based on radiologic findings. While further stratification of mass location could be done, it was deemed out of the scope of this paper’s goal, which was to provide a basis for further research to be performed. All patients were early-stage, and regardless of histology, patients who were diagnosed with NSCLC were included. Patients had primary lung cancer with histology of adenocarcinoma (n=69, 57%) or squamous cell carcinoma/other (n=52, 43%). One patient had confirmed histology of bronchoalveolar cell carcinoma, which was categorized into the squamous cell carcinoma group for statistical purposes. All patients were prescribed definitive SBRT with a dose range of 40–54 Gy, with the majority of patients receiving 50 Gy over five fractions (n=57, 47%). All patient characteristics are summarized in (Table 1).
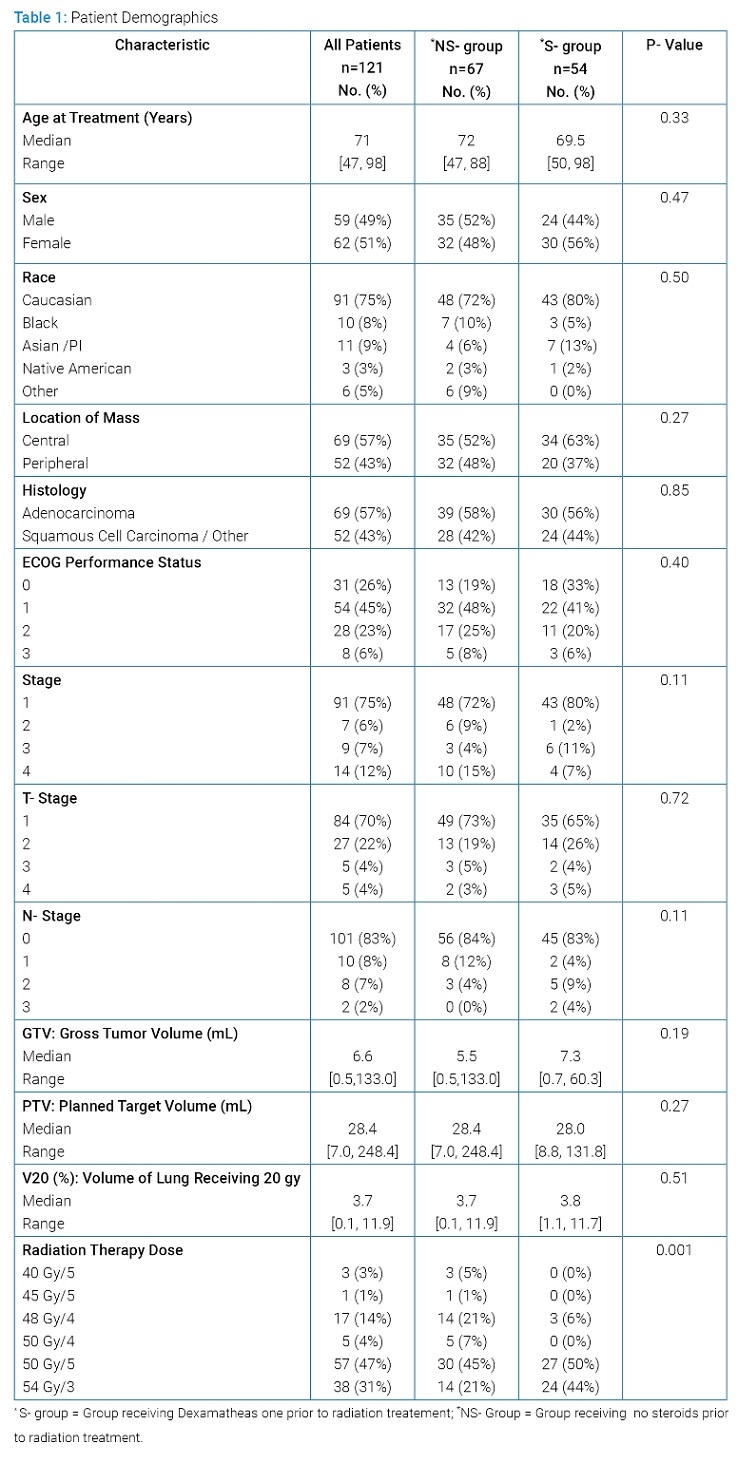
When comparing S-group and NS-group, they were similar in terms of age (P=0.33), sex (P=0.47), race (P=0.5), histology (P=0.85), tumor location (P=0.27), ECOG performance status (P=0.4), cancer staging (P=0.11), and volumetric tumor measurements (GTV, PTV, and V20) (P=0.19, P=0.27, P=0.51, respectively). The NS-group had six patients who identified their race as “other.” S-group received higher radiation doses, with 94% of the group receiving 50–54 Gy as compared to 73% of the NS-group receiving 50–54 Gy (P=0.001).
Radiation Pneumonitis: The main goal of this study was to observe RP events between S-group and NS-group. RP was graded from increasing severity on a scale of 1 through 5. All patients observed in this study showed radiologic evidence of ground-glass opacities or consolidation, indicating at least some degree of radiation changes at the sites of treatment. Because of this, patients who were determined to have asymptomatic Grade 1 RP were compared with symptomatic Grade 2+ RP. In our study population, thirty-one Grade 2+ events, comprised of 22 females and nine males, were observed. In the NS-group, eleven patients experienced Grade 2 RP, 6 experienced Grade 3 RP, and one experienced Grade 5 RP. In the S-group, 12 patients experienced Grade 2 RP, and one experienced Grade 4 RP. There was no statistically significant difference in RP grade between S-group and NS-group. In a logistic regression model adjusting for age, sex, ECOG performance status, staging, radiation dose, and histology, there was no statistical difference between the NS and S groups (Odds Ratio: 0.74; 95% CI (0.3–1.84); P=0.52). Based on this adjusted model, females had increased odds for the development of RP when compared to males (OR 3.78; 95% CI, 1.46–9.76; P=0.01). Overall, Grade 3+ RP events occurred in 8 patients throughout our population (7 patients in the NS-group and one patient in the S-group). This low incidence of Grade 3+ RP toxicity is not surprising based on the decreased volume of tissue involved in SBRT treatments compared to RT. Due to the small number of Grade 3+ toxicities, logistic regression, and adjustment for the variables of interest used in the Grade 2+ analysis was unable to be performed.
Adverse Events: Observing adjuvant steroid therapy’s effect on the incidence of related radiation side effects experienced by S-group and NS-group was an important objective of this study. Incidences of all graded adverse effects are summarized in (Table 2).
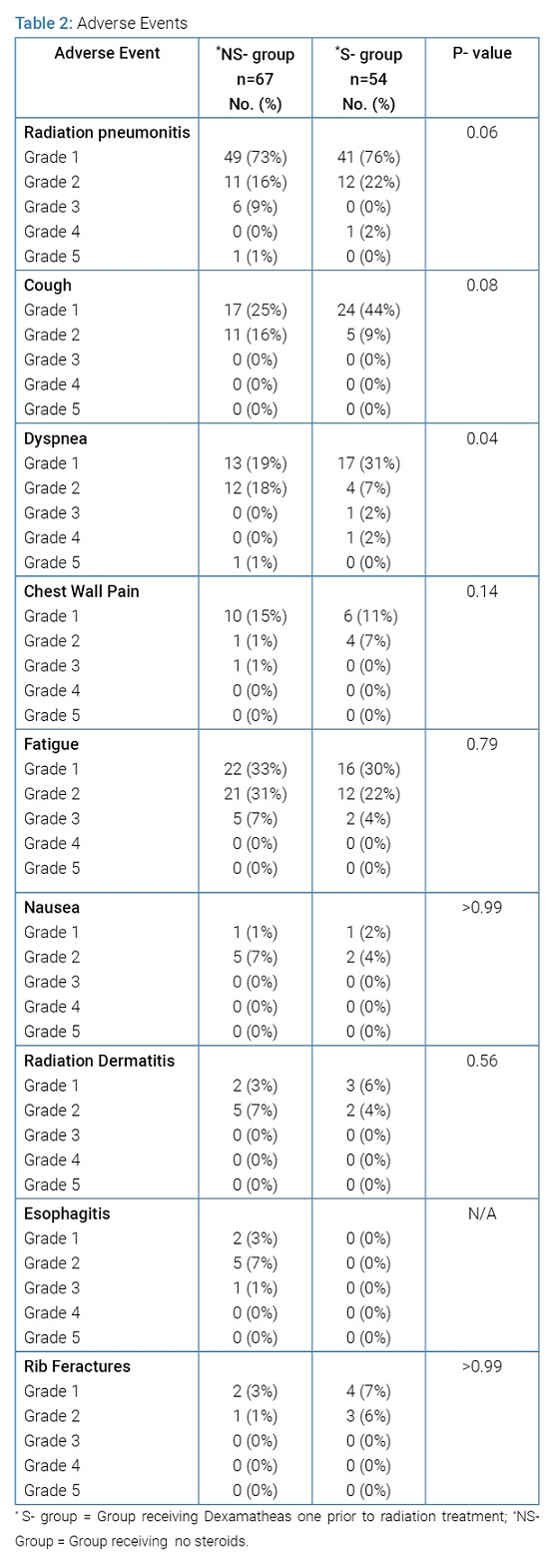
When comparing NS-group and S-group adverse events, both groups were observed not to have a statistically different reporting of cough (P=0.08), chest wall pain (P=0.14), fatigue (P=0.79), nausea (P>0.99), radiation dermatitis (P=0.56), and rib fractures (P>0.99). There was a statistically significant difference across grades of dyspnea between NS-group and S-group (P=0.04). Esophagitis was not observed in the S-group compared to 8 total experiences in the NS-group. Due to the lack of adverse events related to esophagitis in the S-group, a statistical comparison could not be performed. The most commonly experienced adverse effects in the NS-group included fatigue (72%), followed by cough (42%) and dyspnea (39%). In the S-group, the most commonly experienced adverse effects were also fatigue (56%), cough (54%), and dyspnea (43%). When looking at all side effects, eight patients (12%) in the NS group and four patients (8%) in the NS group experienced Grade 3+ side effects.
Discussion
The use of adjuvant steroid therapy varies significantly across the nation because of limited data exploring the significance these regimens have on patient outcomes. Since significant inflammation can be induced by radiation, it is feasible to make the hypothesis that corticosteroids may be an effective measure to combat SBRT adverse events, particularly esophagitis, fatigue, dermatitis, and RP. While empiric steroid use is relatively common among cancer patients, the investigation into adjuvant steroid use with SBRT for NSCLC is extremely limited. As such, caution should be used with steroid therapy due to the potential side effect profile, which can include immunosuppression, hyperglycemia, weight gain, and bone fractures. More data is needed to determine if supplemental steroid therapy with SBRT can improve inflammatory radiation effects without itself promoting dangerous results.
The results by the Chiang and Khan studies provide a starting point for the hypothesis of supplementary steroid treatments. While these studies delivered information about SBRT use in spinal tissue, they showed an association of how steroid use can improve side effects. As seen in Chiang et al., pain flairs due to radiation effects were seen in 68% (28/41) of patients [22]. When rescued with 4 mg doses of dexamethasone, however, pain scores improved dramatically from 6/10 to less than 2/10 (P<0.0001) [22]. Khan et al. expansion of this data provided a glimpse into how prophylactic steroid therapy could be utilized in a population receiving SBRT. Khan’s team found that prophylactic dexamethasone in a spinal SBRT patient population produced a 19% (9/47) incidence of pain flair when compared to Chiang’s population of 68% pain flair incidence (P=<0.0001). In this study, dexamethasone dosing was also explored, with two groups receiving dexamethasone doses of either 4 mg (N=24) or 8 mg (N=23). The incidence of pain flairs between these groups was found not to differ (25% vs. 13%, respectively, P=0.46). Along with pain flairs, functional status was also monitored between groups using the Brief Pain Inventory (BPI). Based on this scoring index, most functional scores showed no differences (P=0.2), with the exception of both walking ability (P=0.005) and relations with others (P=0.035), thus favoring the use of 4 mg dosing [23]. These promising results suggest improvement of side effects with the use of prophylactic steroid regimens as well as an effective dosing pattern. The findings by Chiang and Khan give rise to the possibility of expanding the use of preventative steroid therapy to a broader scope of symptoms and tissues targeted by SBRT. This retrospective study seeks to add to the few findings of prophylactic steroid therapy with SBRT by observing its use in lung cancer patients.
The primary objective of our study was to evaluate whether the addition of steroid use in lung cancer patients receiving SBRT lowered the incidence or severity of RP. Overall, only 31 patients in our population were observed to have mild symptomatic Grade 2 or above RP. This small number of RP incidence is unsurprising, given SBRT’s precision in radiation delivery. Compared to conventional RT, SBRT’s dose deposition accuracy minimizes the volume of healthy lung tissue receiving radiation doses associated with RP incidence (dose = 20 Gy). In our population, dexamethasone was not associated with reducing RP incidences between S-group and NS-group (OR 0.74; 95% CI, 0.3–1.84; P=0.52). In addition, statistical significance was not found when comparing the overall severity of RP experienced by each group (P=0.06). While these endpoints were not statistically significant, the data suggested a trend between the groups. The S-group experienced only 1 Grade 3+ RP event compared to 7 Grade 3+ RP events in the NS-group. Unfortunately, due to the small number of Grade 3+ toxicities, logistic regression, and adjustment for the variables of interest could not be performed. Based on this study’s RP data, this trend could indicate a potential benefit for supplementary steroids to reduce the severity of RP experienced rather than preventing RP. However, an increased sample size is needed to explore this trend in fuller detail. In this study population, one patient experienced fatal Grade 5 RP in the NS-group due to complications with underlying pulmonary fibrosis.
The secondary goals of this study were to observe associations for developing RP and supplemental steroid therapy’s effect on the incidence of radiation-related adverse events. Of the measured associations for developing RP, only female gender was found to increase odds of developing RP grade 2+ when compared to males (OR 3.78; 95% CI, 1.46–9.76; P=0.01). Some studies have proposed an increased RP propensity for women due to differences in total lung volumes and higher incidences of autoimmune disease [26–28]. These studies have proposed that RP may pose a similar hypersensitivity reaction to that of an autoimmune disease, resulting in a higher risk of lung injury for women. A study by Robnett et al. set out to identify factors predictive of RP by observing 148 lung cancer patients. They found the risk for developing RP based on gender was 15% for women compared to 4% for men (P=0.01) [26]. While our data agree with previous studies in regard to increased RP incidence in women, the reason for this difference between sexes remains ambiguous and warrants further study.
When observing steroid therapy’s effect on radiation-related adverse events, both groups were found to have similar side effect profiles with the exception of dyspnea. The S-group showed a significant difference in the severity of dyspnea experienced compared to the NS-group (P=0.04). Six patients in the S-group experienced above mild Grade 1 dyspnea in comparison to 13 patients in the NS-group. Dyspnea, in general, is a particularly challenging and prevalent symptom in lung cancer patients with an estimated average incidence of over 70% [29]. By reducing the additive dyspneic effect of RT, there is potential to maintain or improve patient quality of life. With this study’s findings, there is an association for steroid regimens to reduce dyspnea. Due to the prevalence and distress dyspnea can cause, adjuvant steroid therapy may provide a simple and effective way for physicians to improve care for patients receiving SBRT for lung cancer.
Esophagitis and rib fracture incidence posed interesting results. While eight patients experienced esophagitis in the NS-group, this side effect was not observed in the S-group. In radiation therapy for lung cancers, the esophagus can become involved if the planned tumor volume deposition of radiation overlaps with the esophagus. This typically occurs when the tumor is located more medially within the lung fields near the esophagus. If the tumor volume and amount of surrounding tissue involved in radiation treatment is close and large enough, the esophagus could ultimately be involved in inflammatory events. In our population, however, there was no statistically significant difference between groups when comparing the location (central or peripheral) or tumor volumetrics (PTV, GTV, V20). To fully evaluate this finding, detailed dosimetric and volumetric analysis would need to be done to determine esophageal involvement for each patient in this cohort. The observation of esophagitis differences between groups warrants further study with a larger and more controlled study group.
Seven cases of rib fracture were observed in the S-group compared to 3 cases from the NS-group. While this disparity was not statistically significant, this difference does bring up a potential consideration for supplemental steroid therapy. Steroids themselves have various side effects, including the promotion of osteoclast activity, the reduction of absorption of calcium in the intestines, and increased calcium excretion through the kidneys. Typically, these effects can decrease bone mineral density and cause subsequent osteoporosis in patients taking prolonged courses of steroid therapy. However, when these steroid effects are combined with the deleterious properties of radiation on bone matrix, there may be a synergistic effect that rapidly promotes bone degradation. Because of the long duration needed for steroids to promote osteoporotic changes, further study is needed for both the clinical and statistical relevance of this topic.
This study has several limitations. Our analysis is retrospective in its design, and as a result, selection bias is a confounder of our outcomes. This study population experienced only 31 Grade 2+ RP events between both groups. Because of this small sample size, it is not very easy to draw conclusive decisions about adjuvant steroid therapy’s role in preventing or reducing RP events. Since the overarching purpose of this study was to broadly assess additional steroid therapy’s role in reducing side effects, specific comorbidities were not included as a factor for determining patient eligibility. Only ECOG performance scores were used as a means to assess overall patient status at the time of radiation treatment. In terms of grading radiation-induced side effects, this study used the Common Terminology Criteria for Adverse Events (CTCAE) version 4.02 from the U.S. Department of Health and Human Services as a means to objectively quantify subjective signs.
Finally, the statistically significant difference in radiation dosages between groups was a point of interest in the patient demographics. In the NS-group, only 73% of patients received total doses of = 50 Gy compared to 94% of the S-group (P=0.003). This finding could allude to the tendency for patients to be treated with adjuvant steroids when requiring generally higher doses. This difference could also be a potential confounder. High radiation dose is known to be a predictor for developing RP. Because of the difference in doses received between groups, this could cause the S-group to experience a deceptively higher RP incidence when compared to the NS-group. This could skew the results toward the null hypothesis. A randomized, controlled study comparing similarly dosed groups is warranted to investigate the role of adjuvant steroids further.
The possibility of decreasing radiation side effects by the addition of adjuvant steroid regimens remains a potentially valuable tool for physicians to limit possible radiation toxicities. However, the number of studies addressing the significance of adjuvant steroids for any radiation technique or target tissue is remarkably limited. Overall, this study aims to be one of the first to address the topic of adjuvant steroid treatments formally and inspire further research into a topic that could inexpensively improve quality of life for lung cancer patients receiving radiation.
Conclusion
In this study population, adjuvant steroid therapy was not observed to reduce or prevent RP with statistical significance. However, this study did show that adjuvant steroid use was associated with the incidence of dyspnea. In determining possible predictors for developing RP, females were found to have increased odds of developing RP. Overall, this study observes a potentially beneficial association between adjuvant steroids and the reduction of side effects, as well as identifying a patient population that may be more prone to lung injury. Further research is crucial to determine adjuvant steroid therapy’s role in the reduction of all adverse radiation events as well as further identification of certain populations’ vulnerability to said side effects.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. Cancer J Clin. 2020;70:7–30.
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):359–386.
- Cancer Stat Facts: Lung and Bronchus Cancer. Cancer Stat Facts-SEER. 2017.
- Hartwig MG, D’Amico TA. Thoracoscopic lobectomy: the gold standard for early-stage lung cancer? Ann Thorac Surg. 2010;89(6):2098–2101.
- Lackey A, Donington JS. Surgical Management of Lung Cancer. Semin Intervent Radiol. 2013;30(2):133–140.
- Dosoretz DE, Katin MJ, Blitzer PH, Rubenstein JH, Salenius S, Rashid M, et al. Radiation Therapy in the Management of Medically Inoperable Carcinoma of the Lung: Results and Implications for Future Treatment Strategies. Int J Radiation Oncology Biol Phys. 1992;24(1):3–9.
- Kishan A, Lee P. Radiation Therapy for Stage I Nonoperable or Medically Inoperable Lung Cancer. Semin Respir Crit Care Med. 2016;37:716–726.
- Zhang HX, Yin WB, Zhang LJ, Yang ZY, Zhang ZX, Wang M, et al. Curative radio-therapy of early operable non-small cell lung cancer. Radiother Oncol. 1989;14(2):89–94.
- Timmerman R, Paulus R, Galvin J, Michalski J, Straube W, Bradley J, et al. Stereotactic Body Radiation Therapy for Inoperable Early Stage Lung Cancer. JAMA. 2010;303(11):1070–1076.
- Timmerman RD, Hu C, Michalski J, Straube W, Galvin J, Johnstone D, et al. Long-term Results of RTOG 0236: A Phase II Trial of Stereotactic Body Radiation Therapy (SBRT) in the Treatment of Patients with Medically Inoperable Stage I Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys. 2014;90(1):S30.
- Grutters JP, Kessels AG, Pijls-Johannesma M, De Ruysscher D, Joore MA, Lambin P. Comparison of the effectiveness of radiotherapy with photons, protons and carbon-ions for non-small cell lung cancer: a meta-analysis. Radiother Oncol. 2010;95(1):32–40.
- Grills IS, Mangona VS, Welsh R, Chmielewski G, McInerney E, Martin S, et al. Outcomes after stereotactic lung radiotherapy or wedge resection for stage I non-small-cell lung cancer. J Clin Oncol. 2010;28(6):928–935.
- Nanda RH, Liu Y, Gillespie TW, Mikell JL, Ramalingam SS, Fernandez FG, et al. Stereotactic body radiation therapy versus no treatment for early stage non-small cell lung cancer in medically inoperable elderly patients: A National Cancer Data Base analysis. Cancer. 2015;121(23):4222–4230.
- Shirvani SM, Jiang J, Chang JY, Welsh JW, Gomez DR, Swisher S, et al. Comparative effectiveness of 5 treatment strategies for early-stage non-small cell lung cancer in the elderly. Int J Radiat Oncol Biol Phys. 2012;84(5):1060–1070.
- Faiz SA, Grosu HB, Shannon VR. Pulmonary Complications of Cancer Therapy. In: Kantarjian HM, Wolff RA. Eds. The MD Anderson Manual of Medical Oncology. 3rd ed. New York: McGraw-Hill; 2016.
- Fishman JA. Pulmonary Infection in Immunocompromised Hosts. In: Grippi MA, Elias JA, et al. Eds. Fishman’s Pulmonary Diseases and Disorders. 5th ed. New York: McGraw-Hill; 2015.
- Kishan AU, Wang PC, Sheng K, Yu V, Ruan D, Cao M, et al. Correlation of clinical and dosimetric parameters with radiographic lung injury following stereotactic body radiotherapy. Technol Cancer Res Treat. 2015;14(4):411–418.
- Diot Q, Kavanagh B, Schefter T, Gaspar L, Stuhr K, Miften M. Regional normal lung tissue density changes in patients treated with stereotactic body radiation therapy for lung tumors. Int J Radiat Oncol Biol Phys. 2012; 84(4):1024–1030.
- Kang KH, Okoye CC, Patel RB, Siva S, Biswas T, Ellis RJ, et al. Complication from Stereotactic Body Radiotherapy for Lung Cancer. Cancers. 2015;7(2):981–1004.
- Yamashita H, Nakagawa K, Nakamura N, Koyanagi H, Tago M, Igaki H, et al. Exceptionally high incidence of symptomatic grade 2–5 radiation pneumonitis after stereotactic radiation therapy for lung tumors. Radiat Oncol. 2007;2:21.
- Timmerman R, McGarry R, Yiannoutsos C, Papiez L, Tudor K, DeLuca J, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24(30):4833–4839.
- Chiang A, Zeng L, Zhang L, Lochray F, Korol R, Loblaw A, et al. Pain flare is a common adverse event in steroid-naive patients after spine stereotactic body radiation therapy: a prospective clinical trial. Int J Radiat Oncol Biol Phys. 2013;86(4):638–642.
- Khan L, Chiang A, Zhang L, Thibault I, Bedard G, Wong E, et al. Prophylactic dexamethasone effectively reduces the incidence of pain flare following stereotactic body radiotherapy (SBRT): a prospective observational study. Support Care Cancer. 2015;23(10):2937–2943.
- Edge SB, Compton CC: The American Joint Committee on Cancer: the 7th Edition of the AJCC Cancer Staging Manual and the Future of TNM. Ann Surg Oncol. 2010;17:1471–1474.
- Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655.
- Robnett TJ, Machtay M, Vines EF, McKenna MG, Algazy KM, McKenna WG. Factors predicting severe radiation pneumonitis in patients receiving definitive chemoradiation for lung cancer. Int J Radiat Oncol Biol Phys. 2000;48(1):89–94.
- Das SK, Zhou S, Zhang J, Yin FF, Dewhirst MW, Marks LB. Predicting lung radiotherapy-induced pneumonitis using a model combining parametric Lyman probit with nonparametric decision trees. Int J Radiat Oncol Biol Phys. 2007;68(4):1212–1221.
- Roberts CM, Foulcher E, Zaunders JJ, Bryant DH, Freund J, Cairns D, et al. Radiation pneumonitis: a possible lymphocyte-mediated hypersensitivity reaction. Ann Intern Med. 1993;118(9):696–700.
- Kathiresan G, Clement RF, Sankaranarayanan, MT. Dyspnea in lung cancer patients: a systematic review. Lung Cancer (Auckl). 2010;22(1):141–150.
Keywords
Adjuvant steroid therapy; Stereotactic body radiation therapy; Lung cancer
Cite this article
Monforton AP, Thind BS, Ellbogen CM, Redman MW, Baker KK, Patel SA. Role of adjuvant steroid therapy to limit radiation pneumonitis in stereotactic body radiation therapy (sbrt) treated lung cancer. Clin Case Rep J. 2020;1(2):1–9.
Copyright
© 2020 Shilpen A. Patel. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-4.0).


