Sulcal Artery Syndrome as Atypical Ischemic Complication after Anterior Cervical Discectomy and Fusion: a Case Report
* Valsecchi D;
Brouze IF;
El Rahal A;
Maestretti G;
-
* Valsecchi D: Spine Unit, Department of Orthopedic Surgery, Hôpital Fribourgeois - HFR, University of Fribourg, Fribourg, Switzerland.
-
Brouze IF: Spine Unit, Department of Orthopedic Surgery, Hôpital Fribourgeois - HFR, University of Fribourg, Fribourg, Switzerland.
-
El Rahal A: Spine Unit, Department of Orthopedic Surgery, Hôpital Fribourgeois - HFR, University of Fribourg, Fribourg, Switzerland.
-
Maestretti G: Spine Unit, Department of Orthopedic Surgery, Hôpital Fribourgeois - HFR, University of Fribourg, Fribourg, Switzerland.
-
Aug 17, 2020 |
-
Volume: 1 |
-
Issue: 5 |
-
Views: 4532 |
-
Downloads: 2413 |
Abstract
Anterior Cervical Discectomy and Fusion (ACDF) are frequent interventions. Neurologic worsening and Horner syndrome are known as very rare complications, but none described a Brown-Sequard syndrome as clinical feature of a sulcal artery syndrome. We report a case of a 47-year-old woman who underwent a double level ACDF with plating (C3–C5) and who developed 7–10 hours after the surgery a progressive anesthesia on the right side, with a level on D3, associated with impairment in discriminating heat and cold. On the left side, a hemiparesis with strength M4/5 was assessed. The emergency CT-scan did not show the displacement of the material. The further cerebrocervical MRI showed spinal cord ischemia at the level C3–C4, on the sulcal arteries’ territory. Treatment with oral steroids and acetylsalicylic acid was begun. Two days later, the symptoms worsened with stinging sensation and right hemifacial hypoesthesia on the V2 nerve’s territory. A second cervical MRI showed a progression of the edema, potentially involving the pars caudalis of the trigeminal nerve’s spinal nucleus. We raised the dosage of steroids, which was successful. After multidisciplinary examinations and overall analysis, a diagnosis of incomplete Brown-Sequard syndrome was made, due to ischemia on a ramification coming from a left sulcal artery, without evidence of surgical manipulation or damaging the spinal cord or known underlying risk factors.
Abbreviations
ACDF: Anterior Cervical Discectomy and Fusion; BSS: Brown-Sequard Syndrome; HS: Horner Syndrome; CT: Computed Tomography; MRI: Magnetic Resonance Imaging; PLL: Posterior Longitudinal Ligament; EP: Electrophysiology.
Introduction
The technique known as ACDF is a frequent surgical procedure to treat patients with myelopathy and/or cervical radiculopathy secondary to disc degeneration and/or spondylosis [1]. The complications are rare and often include hoarseness secondary to superior or recurrent laryngeal nerve injury, dysphagia, hematoma, dural tear, esophageal tear, and vertebral artery lesion [1]. Worsening of neurological status and Horner syndrome are very rare and are the most feared adverse event [1,2]. The postoperative neurological worsening is described as tetraparesis, sometime hemiparesis or Horner syndrome (HS), but none has described signs similar to a Brown-Sequard Syndrome (BSS). In the literature, the worsening of the neurological status after cervical spine surgery without clear etiology is most often supposed to be the consequence of reperfusion injury versus hypoperfusion or ischemia. Cybulski described the first post-operatively case in 1988, after a posterior cervical laminectomy [3]. Few articles describe these phenomena, with symptoms close to our case and sometimes resolution under corticosteroid treatment [2,4–6].
In our case, we describe cervical medullary ischemia, presenting with an incomplete BSS and left HS, as clinical manifestation of a Sulcal Artery Syndrome.
Case Presentation
A 47-year-old woman presented at the end of 2019 at our outpatient clinic evaluation with cervical pain lasting for years but with worsening since 2018. The neurological status showed tactile hypoesthesia on the right dermatomes of C3 and C4, a painfully limited mobilization of the neck, Hoffman, Lhermitte, and Spurling signs resulted negatives. No impairment in strength, temperature discrimination, or painful stimulation was assessed. The radiological workup with MRI showed a C3–C4 and C4–C5 disco-arthropathy without cervical stenosis, myelopathy, or radicular impingement. The patient underwent a C3–C4 joints Botox infiltration and sequent denervation, with a positive response but only transitory pain relief. Due to these tests’ outcome, we proposed a surgical treatment by double level ACDF (C3–C4 and C4–C5) with anterior plating C3 to C5 through a left-sided anterior approach, by standard Cloward technique. We performed a continuous neuromonitoring of SSEP and MEP throughout the intervention.
At the 2nd cage’s positioning, on the C4–C5 level, the MEPs on the right upper and lower limb suddenly disappeared. SSEP was normal. The Anesthesiologists didn’t notice any changes in heart rate or O2 saturation; the mean arterial pressure was stable and always maintained between 65 mmHg and 75 mmHg. Although neither the PLL was not opened nor a medullary manipulation performed, an iatrogenic compression was suspected, and the C4–C5 cage was quickly removed; an exploration of the dural surface with the surgical microscope was performed without evidence of any hematoma, cord compression, dural break, CSF leakage or discal fragment. An intravenous administration of 8 mg of dexamethasone was made, the cage replaced with a C3–C5 plate, under fluoroscopic control, with a good result, and the wound closed. The right upper and lower limb MEP remained off. Right SSEP and left-sided SSEP/MEP stayed present. Once awaken, the patient had neither sensitive nor motor loss on the right side. On the left side, a minor deficit (M4/5) in arm abduction was assessed, without any other deficit. A suspicion of EP malfunction was hypothesized.
The patient underwent strict clinical monitoring, without any further symptoms in the first postoperative time. Seven to ten hours after the intervention, the patient progressively developed a left-sided lower limb paresis (M4/5) and, especially, progressive anesthesia at the painful stimulation on the right side, with a level on D3, associated with impairment in the discrimination of heat and cold. We performed an emergency cervical CT-scan that did not show any bony compression or materials dislocation. A subsequently MRI surprisingly showed a medullary left-sided hyperintense signal on the T2-weighted sequences at the C4–C5 level that, despite the artifacts due to the materials, was compatible with an ischemic lesion on the territory of the left sulco comissural arteries (Figure 1).
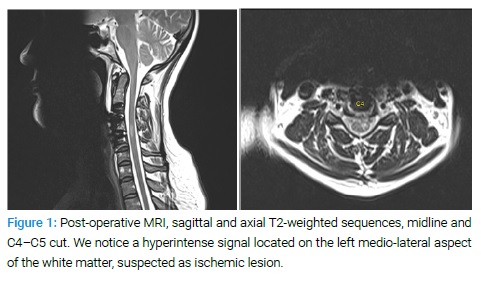
No signs of compressive epidural hematoma, postsurgical medullary contusion on intramedullary bleeding were noticed. The Neurologists were involved in the evaluation, confirming an incomplete BSS and a slightly left miosis and ptosis (HS) signs that typically relate with a sulcal artery syndrome [7]. The treatment with corticosteroid was maintained, and acetylsalicylic acid introduced in the suspicion of acute medullary ischemia. Despite this hypothesis, a cardio-embolic cause of this ischemia was excluded with transthoracic echocardiography, and the patient didn’t report any personal risk factor or positive familiar anamnesis for ischemia.
A new worsening of neurological status with hypoesthesia on the lower right side of the face and itching on tongue and lips was detected two days later. At the new neurological status, left HS disappeared, but the patient showed a potential involvement of the trigeminal nerve, due to the localization on the territory of V2. A new Cerebro-cervical MRI was performed, once again without cerebral lesion but showing the same C4–C5 lesion (Figure 2).
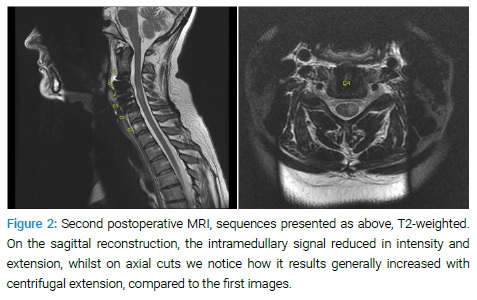
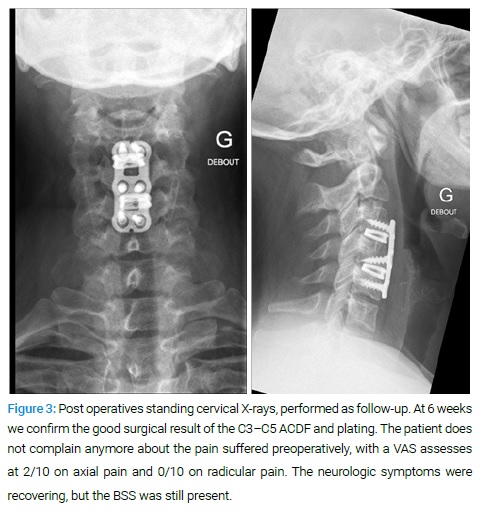
After a multidisciplinary discussion, it was considered likely the anatomical involvement of the pars caudalis of the spinal nucleus of the trigeminal nerve, by the progressing post-ischemic edema. The corticosteroid treatment was increased up to 12 mg per day, for an overall duration of 14 days, associated with 50 mg per day of Pregabalin. The Aspirin was maintained, and the treatment established for three months. The patient showed, seven days later, an almost complete strenght recovery on the upper and lower left limb. The sensitivity stayed diminished with partial recovery on the S1 right level. She was able to walk and discharged home with ambulatory rehabilitation. Follow-up is ongoing (Figure 3).
Discussion
The ACDF’s most common complications are dysphagia, epidural hematoma, and recurrent laryngeal nerve palsy [1]. Cerebrospinal fluid leakage and esophageal perforation are rare [1]. Neurologic worsening and Horner syndrome are very rare [1]. The postoperative neurological worsening is all described as tetraparesis [4,7], paraplegia [6,9], others are hemiparesis [5] and few cases of HS [1,10], but no articles were found describing a BSS.
BSS is an incomplete spinal cord injury caused by damage to one-half of the spinal cord and characterized by loss of sensitivity on touch, pain, and temperature on one side and motor deficit on the opposite side. The lesion may occur on the whole spinal cord, and most cases of BSS result from penetrating trauma or tumors, and only very rarely from acute compression, as a fracture or cervical disc herniation [11,12]. Nevertheless, an incomplete manifestation with less severe symptoms and with good recovery has also been described as clinical feature of a spinal cord ischemia in the territory of the sulcal arteries [7].
The sequent involvement of the right chin, with hypoesthesia on the dermatome of V2, led us to think about an atypical manifestation of the Harlequin's sign, already described as a complication of ACDF [13], but this was ruled out by the absence of flushing and sweating, with only sensory alteration and itching. Therefore, it spoke more for a solicitation of the pars caudalis of the spinal trigeminal nucleus [14], probably due to the perilesional edema, as it was the first symptom relieved by increasing the corticosteroid treatment. The coexisting HS was already described as a synchronic complication of BSS [15].
When postoperative medullary ischemia occurs, two major theories exist about the neurologic worsening. The first and latest theory is the “white cord syndrome,” first described by Chin et al. in 2013 [4]. It is described as ischemia leading to reperfusion lesions, associated with a hyper signal at the postoperative MRI in T2-weighted sequences, in the lesion location. The recommended treatment is either reoperation with steroids or steroids alone. Three other authors also described this theory after an ACDF [4,6,8] and one after a Posterior Cervical Discectomy and Fusion (PCDF) [5].
Nevertheless, the cases described presented evidence of spinal cord compression, and the syndrome is considered a reaction in blood supply changes and reperfusion reaction to the decompression on itself [15]. The second theory is the hypotension-provoked ischemia leading to spinal cord lesion [3,8,16]. Cybulski described the first case in 1988 [3]: his 3 cases report was done without a CT or MRI, but all cases with hypotension episodes showed a neurological deterioration. They do not speak about a possible link with a reperfusion injury component. None of these theories seemed to match with our case, due to the different symptoms and radiological findings described.
The loss of SSEP/MEP during operation in spinal cord injury is also reported in the literature [4,5,8,10]. Daniels et al. [10] reported a management algorithm in case of loss of electro neurophysiologic signal, taken from the work of Ziewacz [18], to provide the best care and treat a potential developing lesion. Nevertheless, when not directly related to intraoperative morphological evidence or a clear neurological deficit at the patient’s awakening, the role and interpretation of these changes are still uncertain. We could consider likely that, as it happens during cerebral vascular surgery [19] and open aortic repair [20,21], combined SSEP and MEP changes may precociously predict a postoperative stroke. Although that, not even the most recent meta-analysis could support or refute intraoperative electrophysiology’s diagnostic value in this specific predictive aim [19].
According to the considerations of above, the most coherent hypothesis was to consider a sulcal artery syndrome as responsible for the symptoms. As seen in the literature, the patients have involvement of the upper cervical spinal cord, with unilateral Brown-Sequard-like presentations with ipsilateral arm and leg weakness, contralateral sensory loss to temperature or hyperalgesia, and minimal or no loss of vibratory sensation and proprioception. A significant improvement at short-term follow-up with minimal or no neurologic deficits is shown, under conservative treatment [7,22]. This syndrome is a very rare nosological entity, with only few case reports in the literature, and mainly seen as complication of vertebral artery dissection or head and neck extensive surgery [7], but it has never been reported as complication of an ACDF, at the moment of the writing.
An eventual underlying anatomical variant that could have weakened the spinal cord to transient hypoperfusion could not even be ruled out. The ischemic lesion was located in the left-sided mediolateral aspect of the cervical spinal cord’s white matter, and it coherently caused the described clinic [15]. Very small arteries supply this region, with a diameter varying between 0.1 mm and 0.06 mm [23].
In our case, the patient lost the MEP on the right side, without any changes in SSEP. The clinical condition in the first hours after the surgery was normal. It was probably the beginning of the lesion, which showed the first neurological signs 7–10 hours later. We could also suppose that it could relate to an incomplete “white cord syndrome”, but our patient didn’t suffer from myelopathy or medullary compression, nor a direct manipulation was performed during the procedure; therefore, this would stay aside from the described condition that usually leads to this syndrome. Moreover, we suppose that the mechanism was ischemia, but there was no perioperative hypotension reported, and, once again, it seems hard to find our “primum movens”. An eventual lack of vascular supply at the moment of the distraction on C4–C5 during the second ACDF, could have led to this hypoperfusion, subsequently evolved into ischemia. However, it cannot be demonstrated via imaging, and angiography performed with genuine scientific interest could implicate several ethics concerns in a patient recovering.
Conclusion
The sulcal artery syndrome is a rare pathology, just occasionally reported as complication after surgery and never associated to an ACDF procedure yet. The pathogenesis is mainly ischemic, but underlying anatomical variants cannot be ruled out.
Maintaining good perfusion pressure throughout a surgical procedure of ACDF is very important, especially when spinal cord exposure and intervertebral distraction are performed. According to this experience, in case of double or more level of ACDF, even if technically and radiologically satisfactory, a distraction on the medullary vascular axe may occur and, despite being usually well-tolerated, a potential exception may happen in patients with underlying anatomical variants or unknown risk factors. Therefore, when feasible, a higher set up of the mean arterial pressure should be discussed with the Anesthesiology, to avoid complication.
The alteration of MEP/SSEP may be the first sign of intraoperative ischemia. Perhaps in this condition, MRI should be the first line indicated imagery and should be considered the opportunity to immediately perform the imaging, in case of persistent loss of potentials at the end of the intervention, although without major clinical signs. Furthermore, maintaining the blood pressure on the level of neural protection even after the surgical procedure, as intensive care procedure, should be similarly considered and discussed in these specific cases, in the aim to prevent neurologic impairment.
A rescue treatment, with corticosteroids and prophylactic acetylsalicylic acid, seems to be effective in similar cases, as also reported in the literature.
Acknowledgements
Thank individuals who contributed to the study or manuscript preparation but do not fulfill all the criteria of authorship.
References
- Fountas KN, Kapsalaki EZ, Nikolakakos LG, Smisson HF, Johnston KW, Grigorian AA, et al. Anterior cervical discectomy and fusion associated complications. Spine (Phila Pa 1976). 2007;32(21):2310–2317.
- Khan MF, Jooma R, Hashmi FA, Raghib MF. Delayed spinal cord infarction following anterior cervical surgical decompression. BMJ Case Rep. 2017;2017. pii: bcr-2017-219863.
- Cybulski GR, D'Angelo CM. Neurological deterioration after laminectomy for spondylotic cervical myeloradiculopathy: the putative role of spinal cord ischaemia. J Neurol Neurosurg Psychiatry. 1988;51(5):717–718.
- Chin KR, Seale J, Cumming V. "White cord syndrome" of acute tetraplegia after anterior cervical decompression and fusion for chronic spinal cord compression: a case report. Case Rep Orthop. 2013;2013:697918.
- Antwi P, Grant R, Kuzmik G, Abbed K. “White cord syndrome” of acute hemiparesis after posterior cervical decompression and fusion for chronic cervical stenosis. World Neurosurgery. 2018;113:33–36.
- Jun DS, Baik JM, Lee SK. A case report: white cord syndrome following anterior cervical discectomy and fusion: importance of prompt diagnosis and treatment. BMC Musculoskelet Disord. 2020;21(1):157.
- Yuebing Li, Donna Jenny, Joshua A, Bemporad, Clarissa J. Liew, John Castaldo. Sulcal artery syndrome after vertebral artery dissection. Journal of Stroke and Cerebrovascular Diseases, Vol. 19, No. 4 (July–August), 2010: pp 333–335, doi: 10.1016/j.jstrokecerebrovasdis.2009.05.006.
- Kalb S, Fakhran S, Dean B, Ross J, Porter RW, Kakarla UK, Ruggieri P, Theodore N, Cervical spinal cord infarction after cervical spine decompressive surgery. World Neurosurg. 2014;81(5–6):810–817.
- Giammalva GR, Maugeri R, Graziano F, Gulì C, Giugno A, Basile L. White cord syndrome after non-contiguous double-level anterior cervical decompression and fusion (ACDF): A "no reflow phenomenon"? [Internet]. Interdisciplinary Neurosurgery. Elsevier; 2016. 2020;14(5). Available from:
https://www.sciencedirect.com/science/article/pii/S2214751916301396 - Daniels AH, Hart RA, Hilibrand AS, Fish DE, Wang JC, Lord EL, et al. Iatrogenic spinal cord injury resulting from cervical spine surgery. Global Spine J. 2017;7(1 Suppl):84S–90S.
- Kim SJ, Lee SH, Bae J, Shin SH. Brown-séquard syndrome caused by acute traumatic cervical disc herniation. Korean J Neurotrauma. 2019;15(2):204–208.
- Wu YT, Ho CW, Chang ST, Chen LC. Brown-sèquard syndrome caused by type iii odontoid fracture: a case report and review of the literature. Spine (Phila Pa 1976). 2010;35(1):E27–E30.
- Fringeli Y, Humm AM, Ansorge A, Maestretti G. Harlequin sign concomitant with horner syndrome after anterior cervical discectomy: a case of intrusion into the cervical sympathetic system. J Neurosurg Spine. 2017;26(6):684–687.
- Neel M. Patel; Joe M Das. Neuroanatomy - spinal trigeminal nucleus. StatPearls Publishing, Treasure Island (FL), 2019.
- Stephen Johnson, Margaret Jones, Jennifer Zumsteg. Brown-séquard syndrome without vascular injury associated with horner's syndrome after a stab injury to the neck. J Spinal Cord Med. 2016; 39(1):111–114.
- Vinodh VP, Rajapathy SK, Sellamuthu P, Kandasamy R. White cord syndrome: a devastating complication of spinal decompression surgery. Surg Neurol Int. 2018;9:136.
- Kaloostian PE, Gokaslan ZL. Cervical spinal cord infarction after decompressive surgery: a closer look. World Neurosurg. 2014;81(5–6):695–697.
- Ziewacz JE, Berven SH, Mummaneni VP, Tsung-Hsi Tu, Akinbo OC, Lyon R, et al. The design, development, and implementation of a checklist for intraoperative, neuromonitoring changes. Neurosurg Focus. 2012;33(5):E11.
- Zhu F, Chui J, Herrick I, Martin J. Intraoperative evoked potential monitoring for detecting cerebral injury during adult aneurysm clipping surgery: a systematic review and meta-analysis of diagnostic test accuracy. BMJ Open. 2019;9(2):e022810.
- Becker DA, McGarvey ML, Rojvirat C, Bavaria JE, Messé SR. Predictors of outcome in patients with spinal cord ichemia after open aortic repair. Neurocrit Care. 2013;18(1):70–74.
- Liu LY, Callahan B, Peterss S, Dumfarth J, Tranquilli M, Ziganshin BA, et al. Neuromonitoring using motor and somatosensory evoked potentials in aortic surgery. J Card Surg. 2016;31(6):383–389.
- Fong JMN, Gee Jin Ng, Tan NCK. Teaching neuroimages: sulcal artery syndrome: a hemicord infarct presenting with incomplete brown-sequard syndrome. Neurology. 2018;90(13):e1177–e1178.
- Santillan A, Nacarino V, Greenberg E, Riina HA, Gobin YP, Patsalides A. Vascular anatomy of the spinal cord. J Neurointerv Surg. 2012;4(1):67–74.
Keywords
Sulcal Artery; Brown-Sequard; Cervical spine; Ischemia; White cord syndrome
Cite this article
Valsecchi D, Brouze IF, El Rahal A, Maestretti G. Sulcal Artery Syndrome as atypical ischemic complication after anterior cervical discectomy and fusion: a case report. Clin Case Rep J. 2020;1(6):1–5.
Copyright
© 2020 Daniele Valsecchi. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-4.0).



