Abstract
Purpose: Patients affected by polypharmacy are at higher risk for significant multi-drug interactions. Inadequate management of these multi-drug interactions in patients discharged from the hospital can lead to readmission and/or medication-related morbidity.
Case: The pharmacist identified drug interactions associated with the cytochrome P450 enzymatic system and P-glycoprotein during a medication safety review for an 86-year-old male. Clinical recommendations were made to mitigate these interactions and align the therapeutic regimen with national consensus guidelines based on patient characteristics. Overall, the pharmacist recommended that the provider deprescribe medications found to be unsafe in the elderly to improve the medication risk score associated with medication-related morbidity.
Conclusion: The interventions recommended by the pharmacist’s interventions decreased the risk of adverse events and may have helped avoid hospital readmission. The case report lends evidence to support a clinical decision support system’s role and its value towards improving patient health outcomes post-hospital discharge.
Introduction
Adverse Drug Events (ADEs) become more common as patients age, and the occurrence doubles for those that are 65 years and older [1]. However, many of these can be mitigated by identifying potential Drug-Drug Interactions (DDIs) [2]. The following case presentation describes a situation in which interventions proposed by a pharmacist during a medication safety review using an advanced clinical decision support system may have significantly improved patient outcomes. These comprise a decrease in the risk of ADEs and hospital readmission through DDI mitigation.
Case Presentation
Patient History: An 86-year-old male with a history of arthritis, asthma, Atrial Fibrillation (AFib), Coronary Artery Disease (CAD), insomnia, Type 2 Diabetes Mellitus (T2DM), Hyperlipidemia (HLD), Hypertension (HTN), and Benign Prostate Hypertrophy (BPH) was readmitted to the hospital cardiac unit after a recent discharge for pneumonia and AFib with rapid ventricular response rate. Upon admission, he complained of coughing that had not resolved since the previous admission nine days prior.
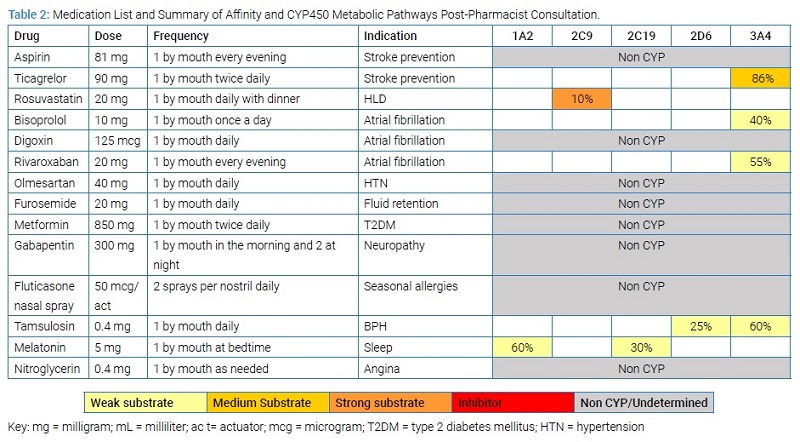
Differential Diagnosis and Treatment: An electrocardiogram showed a left bundle branch block, and the patient’s troponins were elevated with a peak of 1.7 ng/mL. He was also found to have an ejection fraction of 50%. Considering these discoveries, the patient was admitted for Non-ST Segment Elevation Myocardial Infarction (NSTEMI). The creatinine clearance was not decompensated while in the hospital. A percutaneous coronary intervention was performed required two drug-eluting stents. He was also diagnosed with heart failure with preserved ejection fraction (HFpEF). He was treated with diltiazem in the hospital for ventricular rate control, then prescribed triple anticoagulation therapy for one month, and referred to a pharmacist- and nurse- coordinated transition of care program for discharge consultation services. The service included comprehensive medication management services one week and three weeks post-discharge. The pharmacist assessed for DDIs, drug-disease interactions, therapeutic duplications, ADEs, dosing concerns, high-risk medications in the elderly, medication adherence, cost-related barriers, and disease counseling services. See (Table 1) for the list of medications upon discharge prior to the pharmacist’s consultation.
Initial Consultation Outcomes: The clinical pharmacist reconciled the medication list and assessed for clinical appropriateness and medication safety considerations using an advanced clinical decision support system (CDSS; MedWiseTM) within one-week post-discharge. The CDSS allowed the pharmacist to consider multiple medication characteristics, cytochrome P450 interactions, risk of drug-induced long QT syndrome, anticholinergic burden, and sedative burden. Ultimately, the pharmacist aimed to decrease the medication risk score to improve medication safety. First, the pharmacist noted that the patient was prescribed a subtherapeutic dose of liraglutide 0.6 milligrams per day for blood glucose management. Given the patient’s controlled glycosylated hemoglobin level of 6.7%, the pharmacist advised the primary care provider to reassess the need for continued use. Per the package insert, liraglutide is initiated at 0.6 mg for one week to avoid gastrointestinal symptoms; however, it does not provide effective glycemic control at this dose [3].
Instead, the pharmacist advised the patient to follow a healthy diet rich in whole grains, vegetables, and protein. Considering the patient had HFpEF, the pharmacist also recommended a routine walking exercise aligned with provider guidance [4].
Next, given the presence of CHF, the pharmacist also advised the provider to prioritize discontinuation of diltiazem using a tapered schedule over several weeks. Instead, the pharmacist advised the provider to initiate a cardio-selective beta-blocker in a few weeks after the taper, such as bisoprolol, to mitigate the risk of peripheral edema, negative inotropic effects, and mortality associated with diltiazem use [5]. The pharmacist advised the provider to monitor closely for reduced effectiveness of beta-agonist therapy.
Then, upon assessment for high-risk medications in the elderly, the pharmacist advised the primary care provider to consider discontinuation of zolpidem due to its ability to increase the risk of delirium or complex sleep behaviors [6]. Instead, the pharmacist advised the primary care provider to taper off zolpidem and trial low dose melatonin as a safer anti-insomniac alternative, but only if the patient was still experiencing insomnia. Melatonin is known to have a sedative activity; hence, the patient was instructed to be cautious and use precautions if getting out of bed in the middle of the night to prevent falls once they started melatonin treatment.
The patient reported stable blood pressure and heart rate readings during the initial consultation; hence, the pharmacist advised the cardiologist to prescribe sublingual nitroglycerin as needed for angina post-MI [7]. Next, they recommended close monitoring for bleeding complications given the presence of triple therapy. Additionally, they recommended discontinuation of prasugrel and initiation of ticagrelor instead, given the patient’s age and other contraindications [8]. Lastly, the pharmacist recommended an immediate 50% reduction in the maintenance dose of digoxin and close monitoring of digoxin levels given the presence of amiodarone [9].
The pharmacist also recommended immediate discontinuation of atorvastatin, a moderate affinity substrate of CYP3A4, to eliminate the major drug interaction with amiodarone. Instead, the pharmacist advised the provider to initiate rosuvastatin, a moderate affinity substrate of CYP2C9, for secondary prevention. By changing the hydroxymethylglutaryl-coenzyme A reductase inhibitor, the pharmacist aimed to mitigate the risk of ADEs, such as rhabdomyolysis [10]. The pharmacist also recommended gabapentin as a more affordable alternative to pregabalin.
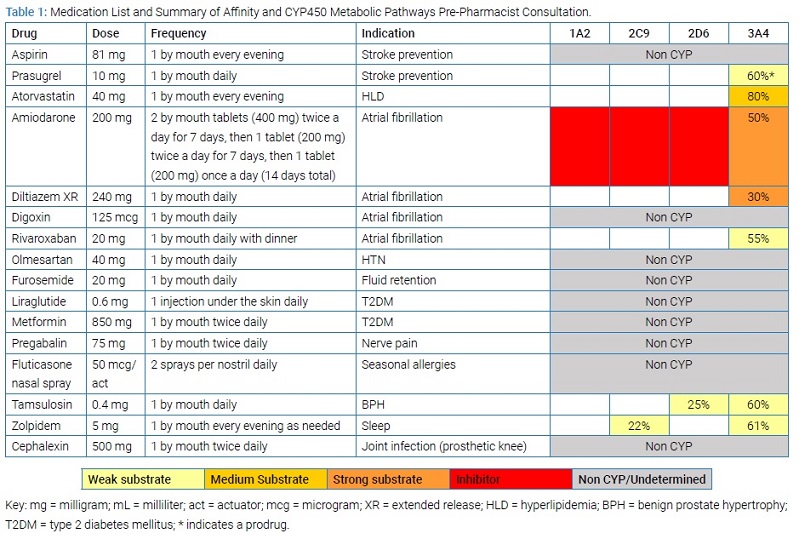
Follow-Up Consultation Outcomes: During a follow-up consultation four weeks later, the pharmacist reconciled the medication list (Table 2).
The provider accepted all of the pharmacist’s recommendations over the course of several weeks.
- Discontinued liraglutide due to optimal biomarkers and implemented lifestyle modifications to manage T2DM.
- Discontinued diltiazem and initiated bisoprolol to mitigate the risk of negative inotropic effects and decreased ventricular ejection fraction.
- Discontinued prasugrel and initiated ticagrelor.
- Added sublingual nitroglycerin.
- Discontinued zolpidem and initiated melatonin instead.
- Discontinued atorvastatin and initiated rosuvastatin instead.
In addition, the provider tapered off amiodarone as ordered on discharge and monitored accordingly. Through these interventions, the MedWiseTM Risk Score [11,12] (medication risk score) decreased in severity from a high-risk score (20) to a low-risk score (11); as this medication score is associated with medication-related ADEs, a decrease in the medication risk score is expected to decrease the risk of ADEs [12].
Discussion
During the initial consultation, the pharmacist identified several drug interactions associated with the cytochrome P450 (CYP) enzymes CYP3A4, CYP2D6, and CYP2C9, and P-gp.
Amiodarone: The anti-arrhythmic amiodarone is metabolized and eliminated through the liver. More specifically, this medication is an inhibitor of multiple CYP450 isoforms, including CYP1A2, CYP2C9, and CYP2D6 [13]. It is also a high-affinity substrate of the CYP3A4 isoform. When the activity of an isoform is inhibited, all other medications metabolized by the same isoform can be affected, which leads to poorer and unintended clinical outcomes [14]. In this scenario, atorvastatin is a substrate of CYP3A4, but amiodarone acts as a competitive inhibitor of CYP3A4. Thus, when taken with amiodarone, atorvastatin is not metabolized as efficiently (decreased clearance), leading to greater concentrations of atorvastatin in the system. Concomitant use of these drugs also would have increased the patient’s risk for myopathy and rhabdomyolysis [9].
Digoxin: The use of digoxin with amiodarone may increase the systemic concentrations of digoxin. Digoxin is a substrate for P-glycoprotein (P-gp), which is an efflux pump responsible for the renal excretion of the drug through tubular secretion [15]. Amiodarone inhibits P-gp, which prevents digoxin from being secreted, leading to increased plasma concentrations [16]. In this scenario, the medication dose prior to the pharmacist’s consultation could have led to digoxin toxicity, given the recent initiation of amiodarone. Hence, it was imperative to closely monitor digoxin levels during concomitant therapy with amiodarone. It is important to note that due to amiodarone’s long half-life, digoxin dose reductions are often required for some time once amiodarone is discontinued [13].
Diltiazem: Similar to amiodarone and atorvastatin, diltiazem is also metabolized by CYP3A4. However, diltiazem has the same affinity as amiodarone for this isoform. In this scenario, the result of the interaction depended on the order in which the patient took the medications. If diltiazem had been administered hours prior to amiodarone, the metabolism of amiodarone would have been lower [17]. Diltiazem and digoxin are both substrates for P-gp. In this scenario, if the patient had administered both medications concomitantly, it would have increased the risk of bradycardia and AV block, which are synergistic cardiac effects shared by both drugs [18–20]. Hence, it was recommended to discontinue diltiazem and prescribe an alternative agent to mitigate these risks.
Prasugrel: Triple therapy consisting of two anti-platelets and one anticoagulant can be used in patients with atrial fibrillation that undergo percutaneous coronary intervention. However, there is evidence that dual antiplatelet therapy plus a direct oral anticoagulant increases the risk of bleeding and is non-superior to a single antiplatelet plus direct oral anticoagulant [21]. Currently, research is being conducted to reassess the duration of antiplatelet therapy and to determine if ticagrelor or prasugrel alone prevents ischemic events, as well as dual-antiplatelet therapy [22]. The antiplatelet drug prasugrel is a prodrug that the body completely hydrolyzes to an inactive intermediate metabolite. That inactive metabolite is then converted to an active metabolite by CYP3A4 and CYP2B6. For this patient, however, the presence of amiodarone and diltiazem would prevent, at least partially, prasugrel from converting to the active metabolite. Thus, the pharmacist recommended discontinuing prasugrel and initiating an alternative antiplatelet, such as ticagrelor, that does not require activation [23].
Ticagrelor: Following the proposed changes in the drug regimen, the pharmacist recommended monitoring for ADEs related to bisoprolol, rivaroxaban, and tamsulosin. These three medications are substrates of CYP3A4. Ticagrelor has the greatest affinity of these three, and dose separation to mitigate this interaction was not feasible, given its twice-daily dosing.
Conclusion
The pharmacist’s clinical recommendations may have helped avoid hospital readmission related to heart block, diabetes, and potentially delirium through interventions designed to mitigate drug interactions associated with medication-related morbidity.
Keywords
Medication safety; Drug interaction; Transition of care; Pharmacist
Cite this article
Tranchina K, Turgeon J, Bingham J. Integrating a novel medication risk score and use of an advanced clinical decision support system into a pharmacist- and nurse-coordinated transition of care program to mitigate drug interactions. Clin Case Rep J. 2021;2(1):1–5.
Copyright
© 2021 Bingham J. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-4.0).