Introduction
Babesiosis is a worldwide emerging tick-borne disease caused by Babesia, a malaria-like parasitic protozoan that invades the Red Blood Cells (RBCs), leading to hemolysis (1). There are over 100 species of Babesia protozoans, several of which are known to commonly cause human infections, including Babesia microti, Babesia divergens, Babesia duncani, Babesia venatorum, and Babesia crassa-like pathogen [1–3]. Most cases of Babesiosis in the United States are attributable to infection with Babesia microti. In the United States, more than 90% of reported cases of Babesia infections occur in Connecticut, Massachusetts, Minnesota, New Jersey, New York, Rhode Island, and Wisconsin – depicting a northeastern and upper midwestern pattern of endemicity [1,4,5]. The annual number of cases reported to CDC in 2018 was 2161, with the highest cases in Massachusetts (527) and New York (641). Babesiosis is not a reportable condition by law in some states, and this might have affected the annual number of cases reported [6]. This distribution pattern is related to the geographic distribution of both the parasite's tick vector (Ixodes scapularis) and its primary reservoir, the white-footed mouse (Peromyscus leucopus) [5]. Babesia microti infection's clinical manifestations range from asymptomatic to mild, moderate, and severe manifestations. The infection is usually asymptomatic to mild in young and healthy individuals but can be severe in elderly and immunocompromised patients [1,7,8].
Among the complications of Babesiosis, splenic infarction has been recognized as a rare complication, but it is life-threatening because of the risk of splenic rupture, hemorrhagic shock, and death [7,9,10]. Microthrombus formation, RBC lysis, cytoadherence of the infected RBCs to the vascular endothelium, and lack of deformability of the RBCs obstruct the vascular flow within the spleen and lead to splenic infarction and necrosis [11]. Splenic infarction does not correlate with the percentage of parasitemia or immune status [8]. We present a case report on multiple splenic infarcts secondary to babesiosis in light of its rare occurrence.
Case Presentation
An 86-year-old man resident of New York State with a past medical history of atrial fibrillation on warfarin for cardioembolic prophylaxis and Diltiazem for rate control presented to the emergency department with a 4-day history of generalized weakness, confusion, fever, chills, and dark urine. The patient had engaged in recent outdoor activity, working on his deck at home for a few days prior to the presentation. He had no known history of recent tick or insect bites, as well as no recent contact with a sick person or recent out-of-state travel. The examination was remarkable for a temperature of 103.1°F and impairment of short-term memory. A review of investigations showed pancytopenia (WBC count of 3,400/mcL, hemoglobin of 10.7 g/dL and platelet count of 46,000/mcL), elevated ALT at 70 IU/L, AST at 137 IU/L, LDH at 611 IU/L, and bilirubin at 1.5 mg/dL, with low haptoglobin of < 15 mg/dL. The fibrinogen level was 424 mg/dL. Urinalysis was positive for bilirubin, blood, and protein. Due to his acute confusion, a CT brain was ordered, which showed no acute intracranial event. Because of his elevated liver enzymes and pancytopenia, a CT abdomen was ordered, which showed splenomegaly measuring 14.8 cm craniocaudally and 13.9 cm x 6.9 cm in axial dimensions, with several wedge-shaped peripheral infarcts (Figure 1).
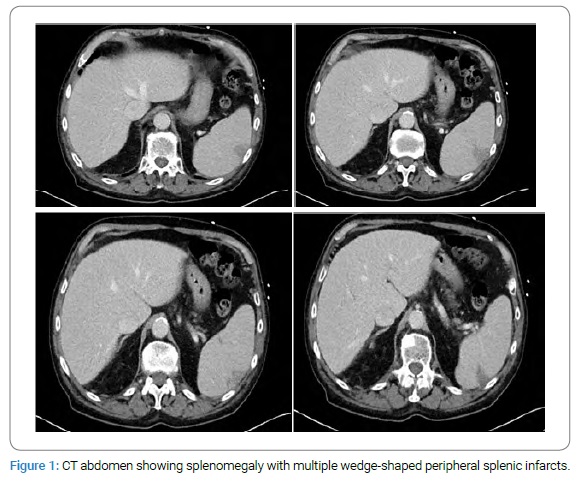
The patient had no prior CT abdomen with which to compare findings. The parasite was seen on the blood parasite screen on presentation. The Babesia microti DNA was detected by RT-PCR. The patient had been on warfarin for anticoagulation based on his history of atrial fibrillation, with an INR of 4.7 at presentation, necessitating the suspension of warfarin. The patient was placed on admission, started on atovaquone 750 mg oral twice daily and azithromycin 500 mg IV daily. He was also placed on doxycycline 100 mg IV twice daily because of possible co-infection with Lyme disease. Blood and urine were sent for culture before starting antimicrobials, both of which yielded no growth after several days of culture. The degree of parasitemia seen on blood parasite smear was estimated at 2.3% after two days on antimicrobials. The initial degree of parasitemia was not obtained before the initiation of treatment.
The general surgical team evaluated the patient for the splenic infarcts, who recommended no surgical intervention. The patient was also evaluated by the hematology/oncology team, who acknowledged the patient’s supratherapeutic INR and agreed with suspending warfarin. Since there was no active bleeding, no Vitamin K or Fresh Frozen Plasma (FFP) was indicated. In addition, for the splenic infarcts, the hematology/oncology team recommended no anticoagulation at the time. For the pancytopenia, there was no indication for granulocyte colony-stimulating factor (G-CSF) or transfusion support. Since the patient was clinically stable from a hematologic standpoint and babesiosis was identified as the cause of pancytopenia, the expectation was that the patient’s hematologic parameters would improve within 3 days–5 days of antimicrobial therapy.
By Day 5 after initiation of antimicrobial therapy, the thrombocytopenia was improving (Platelet 80,000/mcL), and liver enzymes levels were trending down (ALT 65 IU/L and AST 91 IU/L), hemoglobin (9.4 g/dL), and WBC count (3,100/mcL) were stable, the repeated blood parasite smear showed 0.8% parasitemia, and the patient’s INR was 3.0. The patient continued to show both clinical and laboratory improvement. He was no longer confused, his body weakness had improved, he was no longer febrile, and his urine was no longer dark. Screening for Lyme IgM and IgG, as well as Anaplasmosis and Ehrlichiosis antibody titers, were all negative. After six days of therapy, he was discharged with 0.2% of parasitemia and an INR of 2.8. At discharge, warfarin was restarted, and he was sent home with outpatient physical therapy. He was to complete the course of antimicrobial therapy and follow-up within 1 week–2 weeks of discharge with the hematology/oncology team and the infectious disease team.
Discussion
Babesiosis was first reported in cattle having hemoglobinuria in 1888 by Victor Babes [4]. The first human case was identified in 1957 in a young asplenic farmer grazing cattle among pastures infested with ticks; he was found to have a fever, anemia, and hemoglobinuria [5]. Babesia microti is transmitted to humans by the nymphs of the deer tick (Ixodes scapularis). This same tick is responsible for the transmission of Borrelia burgdorferi (the spirochete that causes Lyme disease), Anaplasma phagocytophilum, Ehrlichia species, and in rare instances Powassan encephalitis virus. Babesiosis may present concurrently with other tick-borne illnesses like Lyme disease if the tick also carries Borrelia burgdorferi [12]. Therefore, co-infection with Babesia should be considered in patients diagnosed or treated for Lyme disease and/or human granulocytic anaplasmosis, especially when they present with more severe symptoms than expected or symptoms that persist despite treatment [1]. Cases of transmission of the parasite through blood transfusion and solid organ transplantation have also been reported [13,14].
Severe manifestations of Babesiosis are usually seen in patients ≥ 50-year-old, patients who have had a splenectomy or developed asplenia/hyposplenism, HIV/AIDS, or malignancy, and in patients on immunosuppressive therapy [1,5,15,16]. Overall, only about 5%–10% of Babesiosis cases develop complications; but severe illness has been associated with a higher incidence of complications. For example, the risk of complications could be as high as 20% in immunosuppressed patients or patients who acquire the infection via blood transfusion [16]. Complications that could occur despite appropriate and adequate therapy include acute respiratory failure, disseminated intravascular coagulation, congestive heart failure, liver failure, renal failure, hemophagocytic lymphohistiocytosis, splenic infarction, and splenic rupture [1,5,7,16]. Other severe manifestations of Babesiosis include babesiosis-associated autoimmune hemolytic anemia and immune thrombocytopenia.
Among 34 consecutive patients with babesiosis (mean age of 53.1 years, the age range of 3 months to 85 years) admitted to a hospital in Long Island, NY, acute respiratory failure was the most commonly reported complication, but splenic infarction was not reported [10]. In a systematic review of case reports and case series of Babesia microti infection with splenic complication, the most common presenting symptoms in patients who developed splenic complications were fever (76% of cases), abdominal pain (58% of cases), chills, and malaise (47% of cases), headache (20% of cases), night sweats (14% of cases), and syncope (8.8% of cases); but 42% of patients with splenic complications related to babesiosis did not complain of abdominal pain [17]. The index patient presented in this report did not have abdominal pain.
In a study on Nantucket Island, high levels of parasitemia ( > 10%) and severe anemia (< 10 g/dL) were reported as predictors of the development of complications of Babesiosis and subsequent death [18]. Our patient did not have such high parasitemia at presentation, and his hemoglobin levels were slightly higher than 10 g/dL, yet he had multiple splenic infarcts at presentation. In addition, our patient was relatively healthy and active for his age before symptom onset. He had not been using any immunosuppressive medications, nor did he have a diagnosed malignancy. Considering that severe disease manifestations and subsequent death can be associated with Babesia divergens, Babesia duncani, and Babesia microti infections [5,16], our patient might have developed splenic infarcts as a complication of one of the virulent pathogens. However, because we did not determine the exact species responsible for his illness, we do not have enough evidence supporting this.
In asymptomatic patients, the infection may persist for > 2 years. Asymptomatic patients have no long-term complications, but transmission via transfusion is possible when they donate blood [1]. In patients with mild to moderate disease, common symptoms are fatigue, malaise, weakness, fever, chills, sweating, headache, myalgia, and anorexia – most of these symptoms occurred in our patient. Less frequent babesiosis symptoms are arthralgia, nausea, vomiting, weight loss, abdominal pain, sore throat, dry cough, shortness of breath, photophobia, neck stiffness, and emotional lability. Symptoms usually last 1 week–2 weeks, but fatigue may persist for several months. Common signs in these patients include splenomegaly and hepatomegaly, while less common signs include ecchymoses, petechiae, jaundice, slight pharyngeal erythema, retinopathy with splinter hemorrhages, and retinal infarcts. Lymphadenopathy is usually absent [1,5].
The pathophysiology of splenic infarct and rupture in Babesiosis includes three main mechanisms. Firstly, hemolysis of the RBCs by the invasion of the parasite, leading to endothelial injury, formation of microthrombi, and release of vasoactive factors, resulting in splenic infarcts and necrosis [8]. Secondly, expression of variant erythrocyte antigen 1 (VESA1) on RBCs promotes cytoadherence of the infected RBCs to the vascular endothelium, leading to microvascular obstruction and tissue hypoxia [1,8,11]. Thirdly, the RBCs infected with Babesia and RBCs that express Babesia antigens are removed and destroyed in the splenic red pulp by the splenic macrophages, leading to red pulp hyperplasia evidenced by splenomegaly which can lead to splenic infarct and rupture [11,17]. Although our patient has a history of atrial fibrillation that could also predispose him to thrombus formation in the left atrium, which can lead to splenic infarction, his INR was supra-therapeutic on presentation, which makes cardioembolism as the cause of his splenic infarction less likely. In addition, our patient also presented with acute confusion. The CT brain did not show any acute intracranial event. However, the confusion resolved with the treatment of the Babesia, making the acute confusion likely due to delirium from the underlying acute illness, babesiosis. On presentation, the patient’s fibrinogen was elevated (424 mg/dL), making disseminated intravascular coagulation less likely.
The common laboratory findings in babesiosis consist of thrombocytopenia, normal to decreased white blood cell count, and hemolytic anemia [1,19]. The definitive diagnosis of babesiosis is established by microscopic examination of thin blood smears using Giemsa-stain (Figure 2).
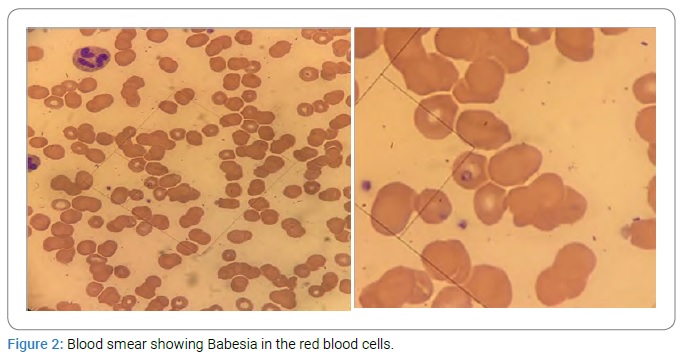
The microscopic findings are the presence of Babesia trophozoites that appear as round or ring forms (which are the most common findings) and the presence of tetrads (“Maltese cross”) in the RBCs [1]. Polymerase Chain Reaction (PCR) and serology can be used to diagnose parasites that cannot be identified by microscopy, and there is high suspicion for the disease [1,5].
The treatment of babesiosis depends on the severity of the disease. In asymptomatic Babesia microti infection, no evidence suggests treatment in these patients [1]. In mild to moderate Babesia microti infection, atovaquone 750 mg oral twice daily plus azithromycin 500 mg oral on day 1, then 250 mg oral daily for 7 days to 10 days are indicated [1,7]. In severe Babesia microti infection, azithromycin 500 mg IV daily plus Atovaquone 750 mg orally twice daily for 7 days–10 days are indicated [1] – this was the antimicrobial therapy our patient received. Six weeks of therapy, including two weeks after parasites are no longer detected on the blood smear, is indicated in immunocompromised patients (HIV/AIDS or patients on immunosuppressive therapy) [1]. An alternative regimen in patients with severe infection is IV clindamycin plus oral quinine [1]. In patients who have ≥ 10% parasitemia, severe anemia (hemoglobin < 10 g/dL), or pulmonary, hepatic, or renal impairment, RBC exchange transfusion is recommended [1,7,20].
In patients with splenic rupture, non-operative management is recommended in the absence of hemoperitoneum because of post-splenectomy severe infection from encapsulated bacteria, Lyme disease, Ehrlichia, and relapse or reinfection with Babesia microti [1,19]. Parasitemia and hematocrit levels are used to monitor response to therapy. In patients not responding to treatment, concurrent tick-borne disease or poor immune response may be considered, and drug resistance may be considered in patients with the relapsing disease [1,12,21].
Our patient resides in New York State, one of the endemic areas of babesiosis in the United States. We treated him with severe babesiosis with IV azithromycin and oral atovaquone because of associated splenic infarcts and his age which put him at high risk for severe infection.
In conclusion, there is currently no vaccine available for the prevention of babesiosis. Patients living in endemic areas and at high risk (elderly patient and immunocompromised) for severe babesiosis, should be counseled to apply tick repellents like diethyltoluamide (DEET) to skin and permethrin to clothing. Wear clothing that covers their upper and lower body during outdoor activities and conduct a thorough examination of their skin after outdoor activities for removal of ticks with tweezers if present [1]. When patients residing in Babesia-endemic areas present with splenic infarcts and hemolytic anemia with pancytopenia, babesiosis should be considered as one of the differential diagnoses. The clinician should consider inpatient management with IV azithromycin and oral atovaquone for elderly or immunocompromised patients presenting with Babesiosis because of the high risk for severe infection. In patients with splenic rupture, non-operative management should be considered in the absence of hemoperitoneum because of the risk of post-splenectomy severe infections [1]. Almost half of the patients with splenic complications related to babesiosis may not complain of abdominal pain [17]; hence, clinicians should consider abdominal imaging to look for splenic involvement in the patients because it can be life-threatening. In patients not responding to treatment, this should raise concern for concurrent tick-borne disease or drug resistance. Lastly, patients should be counseled about the possibility of fatigue persisting even after completing the antibiotic course, and physical therapy should be considered.
Cite this article
Ajibola OA, Ogunleye OO, Collins S, Mazurkiewicz R, Aremu TO, Oluwole OE, et al. Multiple splenic infarcts occurring as a rare complication of babesiosis in an elderly patient on anticoagulation. Clin Case Rep J. 2021;2(3):1–5.


