Optimizing Medications in Patients with Cardiovascular Disease: A Case Report on Unrecognized Prescribing Cascades in Older Adults
Ha M;
Meyer K;
Matos A;
Turgeon J;
* Chandni Bardolia;
-
Ha M: Tabula Rasa HealthCare, Office of Translational Research and Residency Programs, Moorestown, NJ 08057, USA.
-
Meyer K: Tabula Rasa HealthCare, Learning and Development, Moorestown, NJ 08057, USA.
-
Matos A: Tabula Rasa HealthCare, Office of Translational Research and Residency Programs, Moorestown, NJ 08057, USA.
-
Turgeon J: Tabula Rasa HealthCare, Precision Pharmacotherapy Research & Development Institute, Moorestown, NJ 08057, USA; Faculty of Pharmacy, Université de Montréal, Montreal, Quebec H3T 1J4, Canada.
-
* Chandni Bardolia: Tabula Rasa HealthCare, Office of Translational Research and Residency Programs, Moorestown, NJ 08057, USA.
-
Sep 03, 2021 |
-
Volume: 2 |
-
Issue: 3 |
-
Views: 4279 |
-
Downloads: 2715 |
Abstract
Objective: Older adults with cardiovascular diseases are especially prone to polypharmacy due to comorbidities and consequent complexity of medication regimens. Prescribing cascades can occur when a side effect is misinterpreted as a new medical condition. Although the act of deprescribing is an integral part of good prescribing practice, it can be difficult to initiate without surveillance, appropriate communication, and primary care physician advocacy. This case demonstrates how a healthcare team works together to approach and resolve a prescribing cascade in a patient with chronic heart failure.
Case Presentation: An 87-year-old female with chronic heart failure (CHF) was experiencing potential side effects related to polypharmacy. Her long-term diltiazem therapy was identified as a cause of her edema and was deemed no longer appropriate due to the progression of her CHF. The worsening edema led to the prescribing of furosemide, resulting in the need for potassium supplementation. Opportunities to optimize this patient’s medication regimen were recognized as well, including reducing pill burden and assessing the need for additional care based on comorbidities. The patient’s new healthcare team, including the clinical pharmacist and cardiologist, collaborated to resolve the prescribing cascade and create a personalized pharmacotherapy strategy, which resulted in notable improvements in her symptoms.
Conclusion: Resolving prescribing cascades can be a difficult process, as a number of steps and healthcare professionals are often involved. Deprescribing should be a top priority in medication safety, particularly for older adults with cardiovascular disease. Polypharmacy interventions are necessary to encourage safe use and improve patients’ overall wellbeing.
Abbreviations
ADE: Adverse Drug Event; CVD: Cardiovascular Disease; CHF: Chronic Heart Failure; CCB: Calcium-Channel Blocker; HFpEF: Heart Failure with preserved Ejection Fraction; HFrEF: Heart Failure with reduced Ejection Fraction; LVEF: Left Ventricular Ejection Fraction; 25-OHD: 25-O-Hydroxycholecalciferol.
Introduction
Polypharmacy has been defined as the use of more medications than are clinically necessary [1,2]. It is commonly observed in older patients because of their frailty, age-related changes in pharmacokinetics and pharmacodynamics, and the high prevalence of comorbidities [3]. Polypharmacy is correlated with Adverse Drug Events (ADEs) and is problematic for patients and Primary Care Physicians (PCP) when it is overlooked. However, polypharmacy can be prevented with a certain degree of pharmacovigilance [4–6]. While PCPs are often faced with detecting and resolving polypharmacy issues, these issues can be especially challenging in multi-morbid patients, such as those with Cardiovascular Diseases (CVD) [7,8].
In 2016, 48% of adults in the United States were living with CVD, and heart disease was the leading cause of death [9]. According to the American Heart Association (AHA), 69.1% of men and 76.9% of women in the 60–79-year-old age group have CVD, as well as roughly 85% of both men and women in the 80+ years age group [10]. Patients with CVD have a higher chance of experiencing polypharmacy due to CVD-related risk factors such as hypertension, hyperlipidemia, and diabetes, all of which can be treated with medications and result in increased pill burden [11,12]. Although CVD treatment guidelines have allowed for greater standardization of care, they may also have unintended consequences. For example, clinical practice guidelines might not account for competing recommendations across multiple conditions (e.g. recommended use of beta-blockers as first-line therapy for rate control in atrial fibrillation, but cautioned use in patients with diabetes due to effects on glucose metabolism and the masking of hypoglycemic symptoms) [13,14]. Additionally, recommended pharmacological therapies related to CVD typically do not have a specified duration for use, adding a layer of variability [15]. The opportunity for polypharmacy may arise from overlapping pharmacotherapy strategies, as it can be difficult to assess for the unintended consequences of medications if they are indicated for multiple conditions.
Polypharmacy can result from a prescribing cascade, which occurs when a medication-related side effect is misinterpreted as a new medical condition, and a subsequent drug is prescribed to treat the new condition [16–18]. Prescribing cascades have the potential to propagate if left undetected continuously, and ADEs, drug-drug interactions, non-adherence, falls, hospital admission, and mortality are clinical consequences of polypharmacy in older adults [19]. Identifying and resolving prescribing cascades can prevent negative outcomes related to polypharmacy. The three major steps in addressing prescribing cascades are prevention, detection, and resolution [20]. Medication reconciliation processes promote active clinical surveillance and create opportunities to intervene upon potentially inappropriate medications.
This case report will demonstrate the importance of routine medication evaluation to avoid a prescribing cascade. We will also demonstrate how a healthcare team collaborated to resolve a prescribing cascade and optimize the medication profile of a patient with cardiovascular conditions.
Case Presentation
The following information for this case report was collected through available electronic health records and discussions with the patient’s healthcare team. When reviewing the medications of a new patient, a clinical pharmacist identified a medication-related problem involving a potential prescribing cascade. During a medication review call with the patient’s new PCP, the pharmacist discussed this patient’s prescribing cascade and recommended strategies to optimize the patient’s medications.
An 87-year-old female with a documented medical history of atrial fibrillation, chronic heart failure with preserved ejection fraction (HFpEF), hypertension, hypokalemia, vitamin D deficiency, and glaucoma was prescribed a non-dihydropyridine Calcium-Channel Blocker (CCB) (diltiazem), a loop diuretic (furosemide), and potassium supplementation. Her complete list of medications can be found in (Table 1).
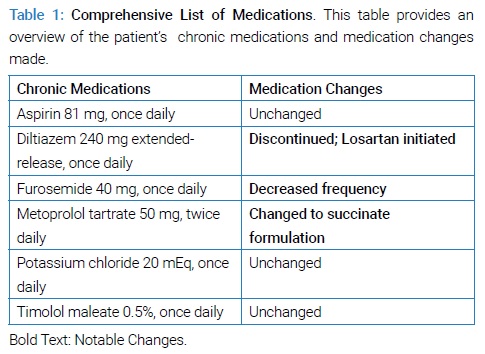
In addition, the nurses on the healthcare team observed that the patient was experiencing moderate pedal edema and had an unstable gait. Medication non-adherence was also documented as an ongoing issue. Additionally, a recent echocardiogram indicated that the patient’s Left Ventricular Ejection Fraction (LVEF) had worsened – although she was originally diagnosed with HFpEF involving diastolic dysfunction, her current LVEF < 40% now indicated heart failure with reduced ejection fraction (HFrEF), a condition associated with systolic dysfunction.
The PCP agreed that the patient’s current issues were likely due to a prescribing cascade related to CCB, a loop diuretic, and potassium supplementation. The PCP also concurred with the clinical pharmacist’s recommendations for optimizing the regimen and prioritizing the patient’s safety. The clinical pharmacist suggested that diltiazem should be discontinued and that the patient’s furosemide and potassium therapies should be adjusted based on the patient’s response to the initial modification. If a CCB was clinically necessary, a dihydropyridine CCB (e.g., amlodipine) should be used instead of diltiazem. The pharmacist also highlighted an opportunity to switch the patient’s rate-control and antihypertensive medication, twice-daily metoprolol tartrate, to the once-daily succinate formulation, as the patient’s blood pressure and heart rate were controlled, in order to help improve her adherence. Lastly, the patient was at high risk of falls due to gait instability and was not taking any form of vitamin D supplementation despite a previous deficiency diagnosis, so the clinical pharmacist recommended re-evaluating the patient’s 25-O-hydroxycholecalciferol levels as a precaution.
The PCP accepted all recommendations and switched the patient from diltiazem to amlodipine for presumed antihypertensive therapy. The PCP then consulted with the patient’s new cardiologist, who also recognized the inappropriateness of the prescribing cascade and that metoprolol succinate would help decrease the patient’s pill burden. The cardiologist clarified that the patient’s CCB therapy was only being used to treat hypertension and suggested that losartan would be more appropriate than amlodipine. Upon follow-up, the PCP noted that the patient adjusted well to the therapy modifications. Blood pressure was well controlled on the new regimen, and the patient was found to have a reduced incidence of pedal edema if any at all.
Discussion
Non-dihydropyridine CCBs, such as diltiazem, are approved (e.g., hypertension, rate-control for atrial fibrillation) and are sometimes used as multimodal therapy [21]. The patient’s progressive CHF, hypertension, and atrial fibrillation created a complex situation that made it difficult to determine the original indication for diltiazem since the patient was new to the healthcare team and presented with an incomplete electronic health record. Because the patient was diagnosed with CHF and reported edema symptoms, it was pertinent to re-evaluate her CHF status. Before the intervention, the patient was categorized as HFpEF. An updated echocardiogram revealed that her CHF had progressed to HFrEF. Generally, non-dihydropyridine CCBs are not recommended in patients with CHF due to negative inotropic effects and adverse cardiovascular events [22]. In fact, the American College of Cardiology (ACC) Foundation and AHA strongly advise against the use of non-dihydropyridine CCBs (i.e., diltiazem) in HFrEF, as this class of medication lacks functional and mortality benefit and can even worsen patient outcomes [23,24].
Furthermore, diltiazem has a dose-dependent relationship with peripheral edema (5%–15% occurrence) [25]. Although amlodipine is also known for dose-dependent peripheral edema, it is the preferred CCB in patients with CHF because it is associated with no difference in all-cause mortality, cardiovascular deaths, or hospitalizations in patients with HFrEF [23,26,27]. Edema resulting from CCBs is not caused by fluid overload, rather by a decrease in arteriolar resistance that goes unmatched in the venous circulation [28]. Initially, the presentation of the patient’s swelling was thought to be the result of the CHF and unrelated to diltiazem. Therefore, furosemide, a loop diuretic, was likely initiated to address this edema. Both loop diuretics and Aldosterone Receptor Antagonists (ARAs) can be used for fluid management in patients with CHF. Unlike ARAs, loop diuretics have mixed evidence supporting benefits in cardiovascular mortality and are mostly utilized to improve symptomatic management of CHF [29]. However, treating patients with clinically inappropriate doses of a loop diuretic increases the risk of over-diuresis. Additional risk factors for over diuresis include falls, urinary incontinence, and electrolyte imbalances [30]. For this patient, potassium supplementation was necessary to counteract the furosemide-induced hypokalemia. Of note, if the patient was hypokalemic and still experiencing edema, an ARA such as spironolactone may have been beneficial for this patient because of its potassium-sparing mechanism.
Although diltiazem is considered a first-line antihypertensive in the AHA/ACC hypertension guidelines, an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker would have been more appropriate according to CHF guidelines [31,32]. As mentioned previously, CVD guidelines may have competing recommendations and often do not account for patients with multiple comorbidities. Further, an increased number of PCPs per patient increases the risk of polypharmacy, so interprofessional collaboration across practices is important for ensuring the safety of patient’s medication regimens. Reaching out to the patient’s cardiologist was key in clarifying the use of CCB therapy, as the cardiologist provided additional perspective on the management of the patient’s HFrEF and hypertension. Communicating the presence of a prescribing cascade to the cardiologist was also crucial, as important patient information can be lost amid transitions of care [33].
Other opportunities to optimize the patient’s medications were addressed as well. Medications requiring multiple daily administration times can add a burden on the patient’s adherence. A literature review found that reducing dosage frequency from multiple administrations to once-daily administration may improve adherence and subsequently result in decreased healthcare costs [34]. In this case, the patient was found to have been taking metoprolol tartrate and had consistent heart rate and blood pressure readings. Switching from metoprolol tartrate twice daily to metoprolol succinate once daily is recommended per CHF guidelines; this change in therapy not only decreases the overall daily pill burden but also may improve patient outcomes [35]. To maintain appropriate rate control for her atrial fibrillation, the patient was monitored closely during the discontinuation of diltiazem and initiation of extended-release metoprolol until she was deemed stable on the new regimen. Additionally, the patient was continued on aspirin for blood clot prevention.
Another health concern arose pertaining to the patient’s untreated vitamin D deficiency. The clinical team took this opportunity to re-evaluate the patient’s vitamin D status and consider supplementation, especially as the patient was reported to have fallen recently. It has been established that low vitamin D levels are associated with falls and fractures, although screening for vitamin D deficiency is no longer recommended if the patient is asymptomatic [36]. Supplementation may be appropriate and may provide benefits since the patient is at high risk of falling, as her physical functioning had been declining with her CHF prognosis [37].
The issues that arose in this patient case were addressed quickly and systematically upon detection. Clinical pharmacists are experts in evaluating appropriate medication use and can initiate the process of identifying and mitigating potential prescribing cascades. A pharmacist’s role in the clinical surveillance of appropriate therapies is valuable to the healthcare team. Had the healthcare teams collaborated during the initial care transition, patient care would not have been disrupted. In this case, all involved healthcare practitioners shared responsibility for patient safety and effectively communicated and collaborated to resolve the medication-related problem. The interprofessional cohesiveness demonstrated in this case report advocates for quality patient care and improved patient outcomes.
Conclusion
A common polypharmacy issue in patients with cardiovascular morbidities is the CCB/loop diuretic/potassium supplement trifecta. Patients with CHF should not be taking non-dihydropyridine CCBs as they can cause ADEs and trigger a prescribing cascade. Not only can these types of cascades negatively impact the patient’s prognosis, but they can also lead to unnecessary pill burden.
The actions of the clinical healthcare team depicted in this case were systematic and required full transparency across various disciplines. These actions made it possible to identify and act upon a prescribing cascade, optimize the patient’s medication regimen to reduce pill burden, and recognize situational risks associated with untreated comorbidities. The clinical team also worked together to survey the risk versus benefit of pharmacotherapy considering the patient’s advanced age and will continue to do so as a part of the patient’s care plan.
Acknowledgments
The authors would like to acknowledge Nishita Shah Amin and Dana Filippoli, for their support in the publication of this case report.
References
- Masnoon N, Shakib S, Kalisch-Ellett L, Caughey GE. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17(1):230.
- Bushardt RL, Massey EB, Simpson TW, Ariail JC, Simpson KN. Polypharmacy: misleading, but manageable. Clin Interv Aging. 2008;3(2):383–389.
- Golchin N, Frank SH, Vince A, Isham L, Meropol SB. Polypharmacy in the elderly. J Res Pharm Pract. 2015;4(2):85–88.
- Lavan AH, Gallagher P. Predicting risk of adverse drug reactions in older adults. Ther Adv Drug Saf. 2016;7(1):11–22.
- Beuscart J-B, Petit S, Gautier S, Wierre P, Balcaen T, Lefebvre J-M, et al. Polypharmacy in older patients: identifying the need for support by a community pharmacist. BMC Geriatr. 2019;19(1):277.
- Dagli RJ, Sharma A. Polypharmacy: a global risk factor for elderly people. J Int Oral Health. 2014;6(6):i-ii.
- Sheikh-Taha M, Asmar M. Polypharmacy and severe potential drug-drug interactions among older adults with cardiovascular disease in the United States. BMC Geriatr. 2021;21(1):233.
- Abolbashari M, Macaulay TE, Whayne TF, Mukherjee D, Saha S. Polypharmacy in Cardiovascular Medicine: Problems and Promises! Cardiovasc Hematol Agents Med Chem. 2017;15(1):31–39.
- Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019;139(10):e56–e528.
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133(4):e38–e360.
- Nobili A, Franchi C, Pasina L, Tettamanti M, Baviera M, Monesi L, et al. Drug utilization and polypharmacy in an Italian elderly population: the EPIFARM-elderly project. Pharmacoepidemiol Drug Saf. 2011;20(5):488–496.
- Hosseini SR, Zabihi A, Jafarian Amiri SR, Bijani A. Polypharmacy among the Elderly. J Midlife Health. 2018;9(2):97–103.
- Tsujimoto T, Sugiyama T, Shapiro MF, Noda M, Kajio H. Risk of cardiovascular events in patients with diabetes mellitus on β-blockers. Hypertension. 2017;70(1):103–110.
- Maan A, Mansour M, J NR, Heist EK. Current Evidence and Recommendations for Rate Control in Atrial Fibrillation. Arrhythm Electrophysiol Rev. 2013;2(1):30–35.
- Krishnaswami A, Steinman MA, Goyal P, Zullo AR, Anderson TS, Birtcher KK, et al. Deprescribing in Older Adults With Cardiovascular Disease. J Am Coll Cardiol. 2019;73(20):2584–2595.
- Ponte ML, Wachs L, Wachs A, Serra HA. Prescribing cascade. A proposed new way to evaluate it. Medicina (B Aires). 2017;77(1):13–16.
- Rochon PA, Gurwitz JH. Optimising drug treatment for elderly people: the prescribing cascade. BMJ. 1997;315(7115):1096–1099.
- Kalisch LM, Caughey GE, Roughead EE, Gilbert AL. The prescribing cascade. 2011;34:162–166.
- Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13(1):57–65.
- Farrell BJ, Jeffs L, Irving H, McCarthy LM. Patient and provider perspectives on the development and resolution of prescribing cascades: a qualitative study. BMC Geriatr. 2020;20(1):368.
- Aronow WS. Hypertension associated with atrial fibrillation. Ann Transl Med. 2017;5(23):457.
- Cheng CP, Pettersson K, Little WC. Effects of felodipine on left ventricular systolic and diastolic performance in congestive heart failure. J Pharmacol Exp Ther. 1994;271(3):1409–1417.
- Elkayam U. Calcium channel blockers in heart failure. Cardiology. 1998;89 Suppl 1:38–46.
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. Circulation. 2013;128(16):e240–e327.
- Diltiazem. In: Lexi-Drugs. Lexi-Comp, Inc. 2020.
- Packer M, O'Connor CM, Ghali JK, Pressler ML, Carson PE, Belkin RN, et al. Effect of amlodipine on morbidity and mortality in severe chronic heart failure. Prospective Randomized Amlodipine Survival Evaluation Study Group. N Engl J Med. 1996;335(15):1107–1114.
- Packer M, Carson P, Elkayam U, Konstam MA, Moe G, O'Connor C, et al. Effect of amlodipine on the survival of patients with severe chronic heart failure due to a nonischemic cardiomyopathy: results of the PRAISE-2 study (prospective randomized amlodipine survival evaluation 2). JACC Heart Fail. 2013;1(4):308–314.
- Sica DA. Calcium channel blocker-related periperal edema: can it be resolved? J Clin Hypertens (Greenwich). 2003;5(4): 291–294,297.
- Faselis C, Arundel C, Patel S, Lam PH, Gottlieb SS, Zile MR, et al. Loop Diuretic Prescription and 30-Day Outcomes in Older Patients with Heart Failure. J Am Coll Cardiol. 2020;76(6):669–679.
- Savage RD, Visentin JD, Bronskill SE, Wang X, Gruneir A, Giannakeas V, et al. Evaluation of a Common Prescribing Cascade of Calcium Channel Blockers and Diuretics in Older Adults With Hypertension. JAMA Intern Med. 2020;180(5):643–651.
- Flack JM, Adekola B. Blood pressure and the new ACC/AHA hypertension guidelines. Trends Cardiovasc Med. 2020;30(3):160–164.
- Inamdar AA, Inamdar AC. Heart Failure: Diagnosis, Management and Utilization. J Clin Med. 2016;5(7):62.
- Mansukhani RP, Bridgeman MB, Candelario D, Eckert LJ. Exploring transitional care: evidence-based strategies for improving provider communication and reducing readmissions. P T. 2015;40(10):690–694.
- Srivastava K, Arora A, Kataria A, Cappelleri JC, Sadosky A, Peterson AM. Impact of reducing dosing frequency on adherence to oral therapies: a literature review and meta-analysis. Patient Prefer Adherence. 2013;7:419–434.
- Tangeman HJ, Patterson JH. Extended-release metoprolol succinate in chronic heart failure. Ann Pharmacother. 2003;37(5):701–710.
- Kahwati LC, LeBlanc E, Weber RP, Giger K, Clark R, Suvada K, et al. Screening for vitamin D deficiency in adults: Updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;325(14):1443–1463.
- Cheema MR, Chaudhry AY. Quality-of-life indicators and falls due to vitamin D deficiency. Int J Gen Med. 2016;9:21–25.
Keywords
Polypharmacy; Prescribing cascades; Chronic heart failure; Calcium channel blockers; Geriatrics
Cite this article
Ha M, Meyer K, Matos A, Turgeon J, Bardolia C. Optimizing medications in patients with cardiovascular disease: a case report on unrecognized prescribing cascades in older adults. Clin Case Rep J. 2021;2(3):1–5.
Copyright
© 2021 Chandni Bardolia. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-4.0).

