Melanoma Metastases versus Neurocysticercosis; the Importance of Correct Interpretation of MR Findings
* Klaudia Soltesova;
Fedicova M;
Gdovinova Z;
-
* Klaudia Soltesova: Department of Neurology, Louis Pasteur University Hospital Kosice, Kosice 04011, Slovakia.
-
Fedicova M: Department of Neurology, Louis Pasteur University Hospital Kosice, Kosice 04011, Slovakia.
-
Gdovinova Z: Faculty of Medicine, Pavol Jozef Šafárik University, Kosice 04001, Slovakia.
-
Dec 10, 2021 |
-
Volume: 2 |
-
Issue: 4 |
-
Views: 7327 |
-
Downloads: 2203 |
Abstract
Magnetic Resonance Imaging (MRI) is very useful for diagnosing brain melanoma (or its metastasis); however, its diagnosis is sometimes challenging. We present a case of a 65-year-old woman admitted to the hospital with an epileptic seizure. Brain MRI revealed multiple lesions, suggestive of chronic granulomas, possibly of parasitic origin, such as neurocysticercosis. The patient then underwent numerous examinations, which did not coincide with neurocysticercosis. Brain biopsy confirmed a melanotic type of solid melanoma metastasis. Oncological treatment was initiated with partial remission.
Nevertheless, based on the last visit (ten months after the start of treatment), the patient developed multiple intramedullary spinal cord metastases. The primary tumor has not yet been found. We retrospectively analyzed the MRI findings and revealed that the initial radiological interpretation of the MRI was incorrect and misleading, which prolonged the diagnostic process. In this case report, we summarize the MRI findings of melanoma metastases (MM). Our work aims to describe the differences between MM and neurocysticercosis on MRI; we also point out that a brain biopsy may be necessary in some cases.
Abbreviations
CNS: Central Nervous System; MM: Melanoma Metastases; MRI: Magnetic Resonance Imaging; CT: Computed Tomography; FLAIR: Fluid-Attenuated Inversion Recovery; DWI: Diffusion-Weighted Imaging; EEG: Electroencephalography; CSF: Cerebrospinal Fluid; ITSS: Intratumoral Susceptibility Signals; SWI: Susceptibility Weighted Imaging.
Introduction
Melanoma represents the third most common tumor entity to metastasize to the Central Nervous System (CNS) after bronchial and mamma carcinoma [1]. CNS metastases occur in ca. 10%−40% of melanoma patients, and according to some sources, the incidence is much higher (up to 75% − detected in autopsy series) [1–3]. Most symptoms of brain MM are nonspecific and depend on the localization of the lesion [4]. We report a case of a 65-year-old patient who presented with a focal epileptic seizure. Brain MM was histologically verified, although the initial brain MRI suggested a parasitic infection (granulomas: mainly due to neurocysticercosis).
Case Presentation
A 65-year-old woman was admitted to an emergency department with the sudden onset of a speech disorder, followed by loss of consciousness and left-sided focal seizures. Her medical history included arterial hypertension, chronic tinnitus, and bronchial asthma. There was no history of any epileptic seizures. Postictal obnubilation with disorientation was observed during the clinical examination, followed by gradual restitution of the clinical condition ad integrum. Urgent brain CT was performed, with findings of multiple hyperdense round lesions (2 mm–15 mm) in the supra- and infratentorial space in the cortico-subcortical localization in both brain hemispheres without apparent perifocal oedema (Figure 1A). On MRI, the lesions were hyperintense on the T1-weighted images (Figure 1B) with homogenous post-contrast gadolinium enhancement (Figure 1C) and hypointense on the T2-weighted (Figure 1D) and FLAIR images (Figure 1E); MR DWI did not reveal greater restriction (Figure 1F).
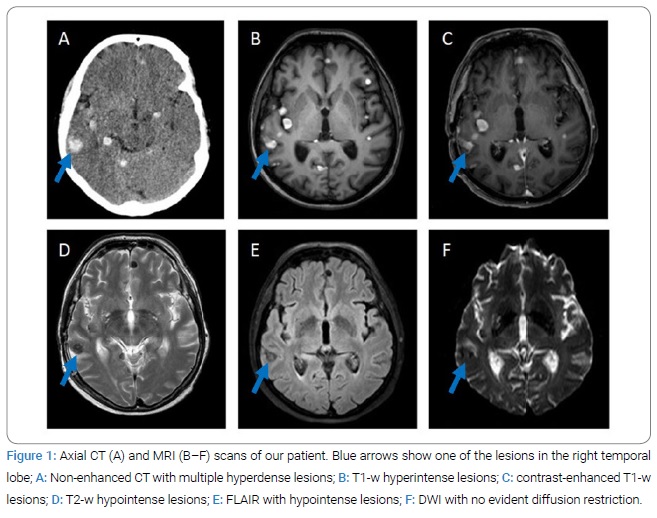
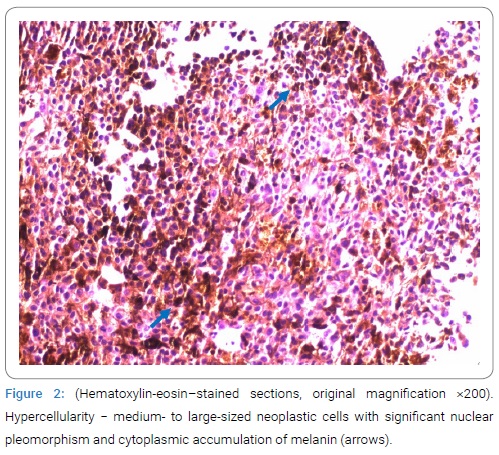
The radiologist evaluated the lesions as chronic granulomas, possibly of parasitic origin, such as neurocysticercosis. Based on this description, serologic test for diagnosis of parasitic infections (Taenia solium, Toxoplasma gondii, Echinococcus granulosus and Echinococcus multilocularis, Leishmania donovani, Toxocara, Trichinella) were performed without relevant results. Subsequently, the patient underwent a number of other examinations. EEG showed a rapid variant of background alpha rhythm with a non-specific abnormality in the right frontal, central and temporal regions. Blood and Cerebrospinal Fluid (CSF) laboratory tests (IgM and IgG for borrelia, CMV, EBV, HSV 1 and 2, HIV, QuantiFERON test) were negative except for leukocytosis. A lumbar puncture was performed to rule out an infectious aetiology; mild pleocytosis and an increased protein level were found [WBC count 40/uL– monocytes (reference values 0/uL–5/uL), total protein 725.70 mmol/L (reference values 150 mmol/L− 450 mmol/L)] and mild elevation of lactate and glucose. The CSF cultivation was negative. As part of the search for a possible oncological aetiology, a chest X-ray, abdominal ultrasound, and whole-body CT were performed, all with a negative result. Of the oncomarkers, CEA showed slightly elevated levels, and other markers (CA19-9, CA125, CYFRA) were normal. Ophthalmological, otorhinolaryngological, dermatological, and gynecological examinations also did not show any relevant abnormalities. After the negative results of the parasitological examination, a neuronavigation-guided open brain biopsy was performed by neurosurgeons, and the melanotic type of solid melanoma metastasis was confirmed by histological examination (Figure 2).
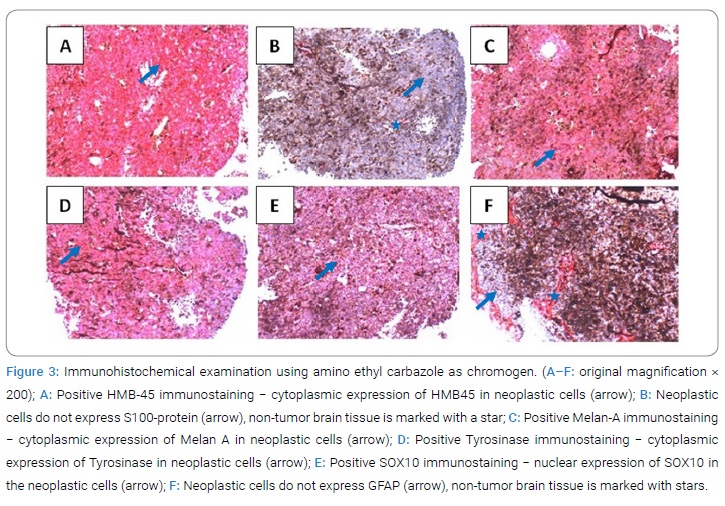
Immunohistochemical analysis revealed the following findings: HMB45+, S100-, MelanA+, Tyrosinase+, SOX10+, GFAP− (Figure 3). No mutation in BRAF V600 was detected in the molecular genetic analysis.
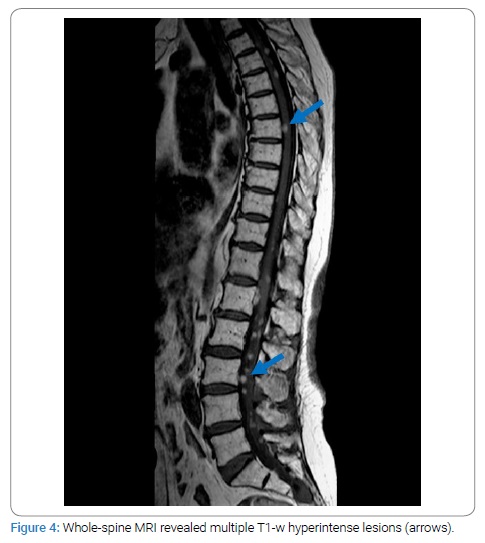
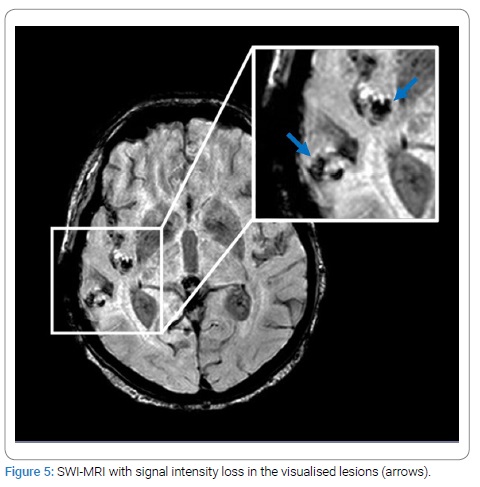
After the patient was discharged from the hospital, palliative oncological treatment was started. It consisted of whole-brain radiotherapy followed by immunotherapy (pembrolizumab) and continued chemotherapy (dacarbazine). Partial remission − a reduction in the growth of brain metastases without the reoccurrence of any neurological symptoms − was achieved with this therapy. Nevertheless, according to the results of neuroimaging techniques at the last visit to the doctor (ten months after the start of therapy), the disease was progressing - the patient had developed multiple intramedullary metastases in the spinal cord (Figure 4). Brain metastases remain unchanged. The SWI sequence was also performed in the last MRI, with characteristic signal distortions in the lesions (Figure 5). Spinal cord radiotherapy is currently being considered. The primary tumor has not yet been found.
Discussion
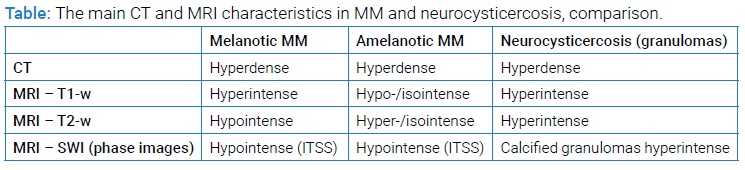
MRI is the gold standard among diagnostic imaging techniques in the early diagnosis and treatment monitoring of patients with brain metastases [5]. However, the interpretation of MRI scans in melanoma can be challenging because brain MM are inherently heterogeneous in their biology, size, distribution, and signal characteristics of melanotic and amelanotic metastases, even on standard T1‐w and T2‐w sequences [1]. For example, 75% of the CNS metastatic melanoma lesions appear on a non-contrast study as increased density; 22% are hypodense, and 3% are isodense. All lesions show contrast enhancement, usually appearing as a homogeneous nodular or ring pattern [4]. The number of melanocytic cells determines the type of MM: melanotic metastases contain more than 10% melanin-containing cells, while amelanotic metastases have less than 10% melanin-containing cells [6]. Melanotic metastases (which represent approximately 24%–54% of cerebral MM) are characterized by high signal intensity on T1-w images and low signal intensity on T2-w images, with no significant post-contrast enhancement. In contrast, MRI findings of amelanotic metastases (ca. 38% of cerebral MM) are non-specific. They are mostly hypo- or isointense on T1-w images and hyper- or isointense on T2-w images. The concentration of melanin in the neoplastic cells affects their signal intensity with a greater amount of melanin. Hence, the greater the high-signal intensity on T1-w images and the greater the low-signal intensity on T2-w images. Diffusion sequences play a minor role in melanoma brain imaging [2]. Isiklar et al. also refer to an indeterminate or mixed pattern with MRI characteristics that do not conform to the previously stated categories and a hematoma pattern with MRI features that exhibit only hematoma characteristics [7].
Melanoma plays a special role regarding susceptibility effects among the malignant entities to metastasize to the brain [5]. Approximately 66% of MM show significant susceptibility artifacts. In tumors, these are referred to as “intratumoral susceptibility signals” (ITSS). A signal distortions due to inhomogeneity in the magnetic field in the tissues are caused by diamagnetic or paramagnetic substances, such as deoxyhemoglobin, iron, or calcification. Because of the melanin content and the tendency to be complicated by hemorrhage, ITSS are much more likely to be seen in MM than other cerebral metastases, in which ITSS are very rare [2,5,8]. Therefore, susceptibility-weighted imaging (SWI) may be very helpful in evaluating possible brain metastases and should be used generally, but especially in patients without known primary malignancy. It was reported that T1w-hyperintense melanotic metastases did not exhibit a higher frequency of SWI signal losses than amelanotic metastases [5]. Melanin content alone reflects the T1-hyperintensity but does not result in a diagnostically relevant susceptibility effect on SWI. The signal distortion is influenced more by secondary phenomena, such as micro hemorrhage rather than melanin content. Therefore, the appearance of melanotic and amelanotic MM on SWI is usually similar, and the type of MM cannot be distinguished only from the SWI images [1,5].
In the differential diagnosis of our case, we took into account neurocysticercosis and other potential parasitic diseases mentioned in the radiological description of the MR scans. Neurocysticercosis is a CNS infection caused by the pork parasite Taenia solium. Cysticercosis is endemic in many developing countries. Due to people’s increased migration to developed countries, cysticercosis has gained global significance [9]. Cysticerci can be located in the brain parenchyma, subarachnoid space, ventricles, and rarely in the spinal cord. Based on the stage and radiological findings, the Escobar classification of parenchymal neurocysticercosis describes five stages: non-cystic, vesicular, colloidal vesicular, granular nodular, and calcified nodular, with different manifestation and presentation in the imaging techniques. Multiple lesions in different stages of development are commonly found. The non-cystic stage can be detected only by laboratory tests, and imaging studies cannot detect them. The invaginated larva has a transparent fluid-filled “vesicle” surrounded by a thin transparent membrane in the vesicular stage. The lesions are hyperdense on CT and iso- to hyperintense on T1-w and T2-w MR-sequences. The scolex was seen eccentrically within the lesion (appearance described as a “cyst with a dot”), which is pathognomonic at this stage. In the colloidal vesicular stage, the parasite undergoes degenerative changes either due to aging or treatment. The cyst degenerates, and the cyst fluid is extruded, which causes an inflammatory reaction with a breakdown of the blood-brain barrier. On MRI, the cyst is hyperintense on T1-w and T2-w images and shows positive post-contrast enhancement. In this stage, the scolex may or may not be seen. The cyst later transforms into a granulomatous lesion by shrinking in size and thickening its walls. The imaging findings in this granular nodular stage are similar to the previous one. The granules in the cyst undergo a process of calcification and the last stage – calcified nodular – can be better seen on CT as hyperdense lesions [9–11]. For identifying calcified lesions on MRI, the SWI sequence can also be helpful. SWI is a sequence that uses magnitude and filtered-phase information. Susceptibility is altered by paramagnetic (hemosiderin, deoxyhemoglobin) and diamagnetic (calcifications) materials. On the magnitude images, both – paramagnetic and diamagnetic materials demonstrate signal drop out and blooming and appear hypointense. The materials show opposite signal intensities on the phase images – paramagnetic materials show hypointensity, while diamagnetic materials appear hyperintense [1,5,12].
In our patient, the hyperintensity and hypointensity, the absence of a visible scolex in the lesions, no abnormalities in the patient’s epidemiological situation, her ethnicity, and no evidence about any possible contamination, as well as the negative anti-cysticercal antibodies, all testified against the diagnosis of neurocysticercosis. The lesions were hyperdense on the non-contrast CT; on MRI, they appeared hyperintense on the T1-w images and hypointense on the T2-w and FLAIR images, with homogenous post-contrast enhancement. This pattern is characteristic of the melanotic type of MM and was incorrectly evaluated by a radiologist. In addition, the SWI sequence, which may have helped us make an earlier diagnosis, was not performed. The pattern of lesions was regular and homogenous, well-circumscribed, and there was no zone of peritumoral oedema − this could also have led to an initial misinterpretation of the MR findings and not considering malignancy. The patient did not have a known melanoma or other tumors, nor did we find other possible metastases that could potentially support the MM hypothesis in an overall differential-diagnostic process; therefore, the final diagnosis was first determined after obtaining the histological results from the brain biopsy.
Based on literature data and our experience, we summarize the differences between MM and neurocysticercosis in the neuroimaging modalities in (Table). As mentioned above, the imaging characteristics are not always sufficiently specific, and they can be challenging to interpret in some instances; we show here the most common manifestations. We present the granular stage in neurocysticercosis, as chronic brain granulomas usually represent a significant problem in differential diagnosis to the metastatic disease.
Conclusion
In summary, we would like to point out some MR findings characteristic for MM which may help to differentiate them from other brain lesions:
- First, circa half of MM shows high signal intensity on T1-w images (because of blood products and melanin), whereas other cerebral metastases rarely demonstrate T1 hyperintensity.
- Second, there is no diffusion restriction described in MM.
- Third, intratumoral hemorrhage is seen much more often in MM than in other brain metastases.
- Finally, ITSS are the SWI is more common in MM than other metastatic entities.
Acknowledgement
We thank Dr. Hribikova from Department of Pathology, Louis Pasteur University Hospital Kosice, for providing materials from the histological studies and her help with interpretation of the findings.
Author contributions: Concept of the work: Soltesova K, Fedicova M, Gdovinova Z; Writing of the first draft: Soltesova K; Review and Critique: Fedicova M, Gdovinova Z; Approved the last version: Soltesova K, Fedicova M, Gdovinova Z.
Ethical approval: No Statement of Ethics Approval from the ethics committee was required for this publication.
The patient declared consent to the publication of her case, including the publication of images.
Funding sources: The authors received no funding support for the publication of this case report.
Conflict of Interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Informed consent was obtained for this publication.
References
- Schwarz D, Niederle T, Münch P, Hielscher T, Hassel JC, Schlemmer HP, et al. Susceptibility-weighted imaging in malignant melanoma brain metastasis. J Magn Reson Imaging. 2019;50(4):1251–1259.
- Breckwoldt M, Bendszus M. [Cerebral MR imaging of malignant melanoma]. Radiologe. 2015;55(2):113–119.
- Oliva IG, Tawbi H, Davies MA. Melanoma Brain Metastases: Current Areas of Investigation and Future Directions. Cancer J. 2017;23(1):68–74.
- Goulart CR, Mattei TA, Ramina R. Cerebral melanoma metastases: a critical review on diagnostic methods and therapeutic options. ISRN Surg. 2011;2011:276908.
- Schwarz D, Bendszus M, Breckwoldt MO. Clinical Value of Susceptibility Weighted Imaging of Brain Metastases. Front Neurol. 2020;11:55.
- Orton T, Gaillard F. Intracranial metastatic melanoma. Radiopaedia. 2021.
- Isiklar I, Leeds NE, Fuller GN, Kumar AJ. Intracranial metastatic melanoma: correlation between MR imaging characteristics and melanin content. AJR Am J Roentgenol. 1995;165(6):1503–1512.
- Villanueva-Meyer JE, Mabray MC, Cha S. Current Clinical Brain Tumor Imaging. Neurosurgery. 2017;81(3):397–415.
- Venkat B, Aggarwal N, Makhaik S, Sood R. A comprehensive review of imaging findings in human cysticercosis. Jpn J Radiol. 2016;34(4):241–257.
- Gaillard F. Neurocysticercosis. Radiopaedia. 2021.
- Oprişan A, Popescu BO. Intracranial cysts: an imagery diagnostic challenge. Scientific World Journal. 2013;2013:172154.
- Mittal S, Wu Z, Neelavalli J, Haacke EM. Susceptibility-weighted imaging: technical aspects and clinical applications, part 2. AJNR Am J Neuroradiol. 2009;30(2):232–252.
Keywords
Melanoma metastases; Neurocysticercosis; Intratumoral susceptibility signals (ITSS)
Cite this article
Soltesova K, Fedicova M, Gdovinova Z. Melanoma metastases versus neurocysticercosis; the importance of correct interpretation of MR findings. Clin Case Rep J. 2021;2(4):1–6.
Copyright
© 2021 Klaudia Soltesova. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-4.0).






