Clinical Value of Bioimpedance during Long-Term Cancer Therapy
* Steven Brantlov;
Lars Jødal;
Berit L Heitmann;
Leigh C Ward;
-
* Steven Brantlov: Department of Procurement & Clinical Engineering, Central Denmark Region, Aarhus N, 8200, Denmark.
-
Lars Jødal: Department of Nuclear Medicine, Aalborg University Hospital, Aalborg 9000, Denmark.
-
Berit L Heitmann: The Parker Institute, Bispebjerg and Frederiksberg Hospital, Copenhagen University Hospital, Denmark; Department of Public Health, Section for General Practice, University of Copenhagen, Denmark.
-
Leigh C Ward: School of Chemistry and Molecular Biosciences, The University of Queensland, Brisbane, Australia.
-
Dec 14, 2021 |
-
Volume: 2 |
-
Issue: 5 |
-
Views: 3297 |
-
Downloads: 2101 |
Abstract
This case report describes the use of bioimpedance spectroscopy (BIS) to monitor changes in hydration status and cellular function in a 73-year-old male patient with non-small cell lung cancer (NSCLC) undergoing a 12-week immunotherapy course. The parameters monitored were phase angle (PA), impedance ratio (IR), and cell membrane capacitance (Cm).
At week 1, the patient weighed 72.6 kg and with a PA of 4.2 degrees, an IR of 0.85 (no unit), and a Cm of 1.21 nanofarad (nF), all outside reference ranges. Throughout treatment, biompedance parameters were measured weekly. By week 12, body-weight had increased to 75.3 kg, PA to 4.8 degrees, IR to 0.82, and Cm to 2.01 nF, within or close to the mean ± SD ranges seen in healthy controls.
BIS can non-invasively provide measurements of changes in hydration status and cellular function on a routine basis in the clinical setting. Continuous monitoring of these parameters may be useful as an adjunct to assess response to immunotherapy treatment of cancer patients. However, to assess the full clinical potential of these parameters in patients with NSCLC and generally, in patients with cancer, a study with a larger number of participants is required.
Introduction
Lung cancer is a leading cause of cancer-related deaths in men and women worldwide [1]. In the oncology community, there is a growing interest in understanding how body composition measures can improve cancer treatment and survival for the 18.1 million individuals diagnosed with cancer every year [2]. Since there is no “gold standard” for body composition determination in cancer patients [3], clinicians typically use body-weight and body mass index (BMI) routinely in the clinical setting. However, they are recognized as being insufficient as measures of body composition change [2]. A recognized problem in cancer patients is the rapid loss of fat-free mass (FFM), a loss that is linked to morbidity following cancer treatment [4], the effect of cancer treatment [5], and survival [6,7]. Loss of FFM may be masked by stable body-weight or weight gain [8], and in cancer patients experiencing treatment-related body-weight loss, body-weight gain during or after recovery may be characterized by an increase in fat mass (FM) rather than FFM [9].
One method that may prove useful for routine body composition determination in cancer patients is bioelectrical impedance analysis (BIA). BIA is a commonly used method in clinical studies since it is non-invasive, inexpensive, simple, quick, and uses portable instrumentation [10]. BIA uses prediction equations to estimate body composition parameters such as FFM and FM. However, these equations are often limited by the general assumption that the composition and density of the FFM are stable among subjects, which is generally untrue due to vast differences in age, sex, ethnicity, and clinical state, e.g., cancer [11]. Consequently, the scientific community has a growing interest in using BIA parameters based on raw impedance data as alternatives to prediction equations [12–15].
In brief, BIA measures the opposition (impedance, Z) to an applied alternating current while passing through the body. Impedance consists of two components: resistance (R), which is the opposition to the flow of current through intra- and extracellular ionic solutions, and capacitive reactance (XC), which is caused by the capacitance of cell membranes and tissue interfaces. In addition, cell membrane capacitance is also reflected by the phase angle (PA) due to the delay between voltage and current flow through tissues.
Phase angle (PA) is the most commonly used raw impedance parameter [16] and is regarded as a biological marker of cellular health, reflecting body cell mass (BCM), membrane integrity, and the hydration status of FFM [17,18]. In various patient groups, the parameter has proven to predict later morbidity and mortality [10,19] and is considered a surrogate for FFM [20]. A second parameter is impedance ratio (IR), the ratio of resistances at different current frequencies, which has been proposed as a potential indicator of edema, pre-clinical cardiovascular disease [21], and overall health [12], a predictor of mortality in hemodialysis patients [22] and to reflect the fluid distribution between the intra- (ICW) and the extracellular water (ECW) [12]. A third parameter is cell membrane capacitance (Cm), a parameter that is directly linked to cell membrane function [23], but has been studied only to a limited extent [14,24,25].
This case report describes the use of BIA raw bioimpedance parameters as indices of changes in hydration status (PA, IR) and cell membrane function (PA, Cm) in a cancer patient following a 12-week immunotherapy course.
Case Presentation
The patient was a 73-year-old male smoker (height = 1.89 m) admitted for lung examination in October 2019 at The Department of Respiratory Diseases and Allergy at Aarhus University Hospital, Denmark, because of prolonged cough and mild cough fatigue. In November 2019, the patient was diagnosed with non-small cell lung cancer (NSCLC) in the left lung lobe, metastases in the right kidney and the pancreas, as well as in mediastinal lymph nodes. Furthermore, the patient was informed that the disease was incurable but that the cancer could be treated to help manage symptoms or slow the progression of the disease.
From November 2019 to July 2020, the patient received three different types of treatments, as described below, at the Department of Oncology, Aarhus University Hospital, Denmark. The effect of each treatment was followed-up by Computer Tomography (CT) scans of the thorax and the upper abdomen and blood tests.
The patient’s first treatment was Alectinib (Alecensa, Roche Holding AG), which is used for patients with anaplastic lymphoma kinase (ALK +), NSCLC that has spread to other parts of the body. After a 12-week course, the treatment was not successful, as the disease had progressed partly concerning the lung’s primary tumor and lymph nodes in the mediastinum and the right kidney tumor and metastasis in the pancreas. In addition, two new metastases in the right adrenal gland and two further metastases in the right kidney had appeared. No liver or bone metastases.
The patient’s second treatment was chemotherapy given in a combination of Carboplatin given IV (Accord Healthcare Limited, UK) and Vinorelbine given as capsules (Accord Healthcare Limited, UK) in a 12-week course. After completing treatment, CT scans showed a further progression of the disease in the places described above.
Thirdly, as a consequence of the lack of effect from the two previous treatments, the patient was admitted to a 12-week immunotherapy course with Pembrolizumab (Keytruda, Merck Sharp, and Dohme BV) given every three weeks (week 1, week 4, week 7, and week 10). Results from CT scans indicated that the treatment had a positive effect on the cancer, showing that the disease was at rest, no new cancer had occurred, and there were places where cancer had been reduced in the size of the nodules.
A Xitron 4200, HYDRA bioimpedance spectroscopy (BIS) device (Xitron Technologies, San Diego, CA, USA), was used for whole-body impedance measurements (taken in triplicate), with skin surface electrodes located at the right wrist and ankle as described previously [26]. Body-weight was measured wearing underpants and a t-shirt on a digital scale. All measurements were performed by SB, who has extensive experience with the bioimpedance technique. Measured bioimpedance data were analyzed according to Cole theory [27] using Bioimp Version 5.4.0.3 (ImpediMed, Brisbane QLD, Australia). The raw bioimpedance parameters provided were:
- Resistance (ohm) at zero (RE) and infinite frequencies (RINF); indices for ECW and total body water, respectively.
- PA50 (degrees), the phase angle at the standard frequency of 50 kHz, calculated from the resistance (R50) and capacitive reactance (Xc50) and measured at this frequency.
- IR200/5 (no unit), calculated as the ratio between resistances measured at frequencies of 200 (R200) and 5 kHz (R5), which is the standard pair of frequencies for IR reporting.
- Cm (nanofarad, nF), calculated from RE (resistance of the ECW), RI (resistance of the ICW), and at the so-called characteristic frequency (ƒc), the frequency of maximum capacitive reactance (XC).
Data measured in the patient were compared with data available [21] for a cohort of 206 healthy Danish males (mean age: 71.7 years, height = 172.2 ± 6.3 cm, weight = 79.1 ± 11.6 kg, BMI = 26.6 ± 3.5 kg/cm2) and reference values from the literature [28,29], which matched the patient’s age and sex.
The case report was performed in accordance with the Helsinki Declaration and was approved by the Central Denmark Region Committees on Health Research Ethics (case number: 1-10-72-181-20) and the Danish Data Protection Office (case number: 2015-41-3942).
(Table 1) summarizes patient characteristics and blood tests collected during the immunotherapy course. The patient presented with historically low body-weight (72.6 kg) at week 1, no energy, no appetite, weakness, and paleness. Three months of clinical care led to an increase of 3 kg in body-weight, an improvement in energy level and appetite, and a return of normal skin color; however, without being cured of the cancer. These changes were reflected by improvements in the patient’s blood test results, notably hemoglobin and leucocyte concentrations (Table 1), and a general reduction of the cancer burden shown by follow-up CT scans.
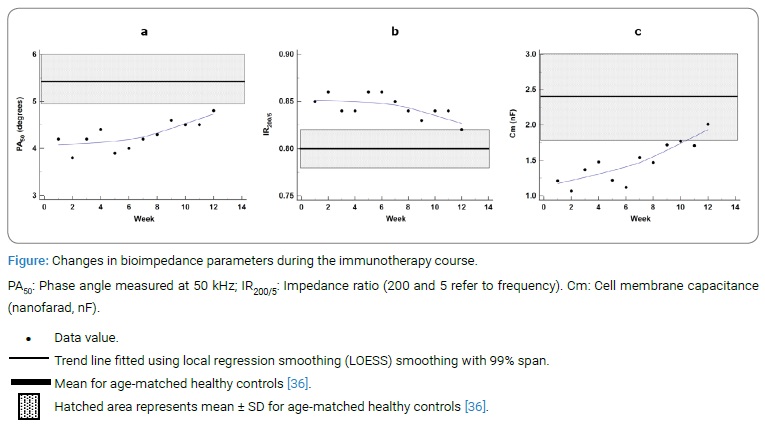
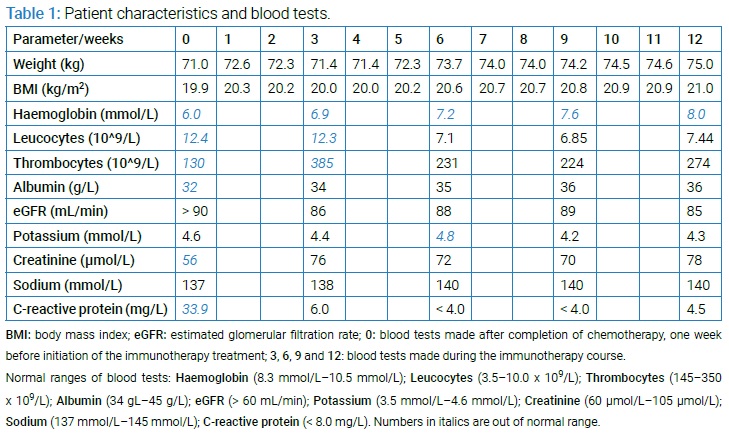
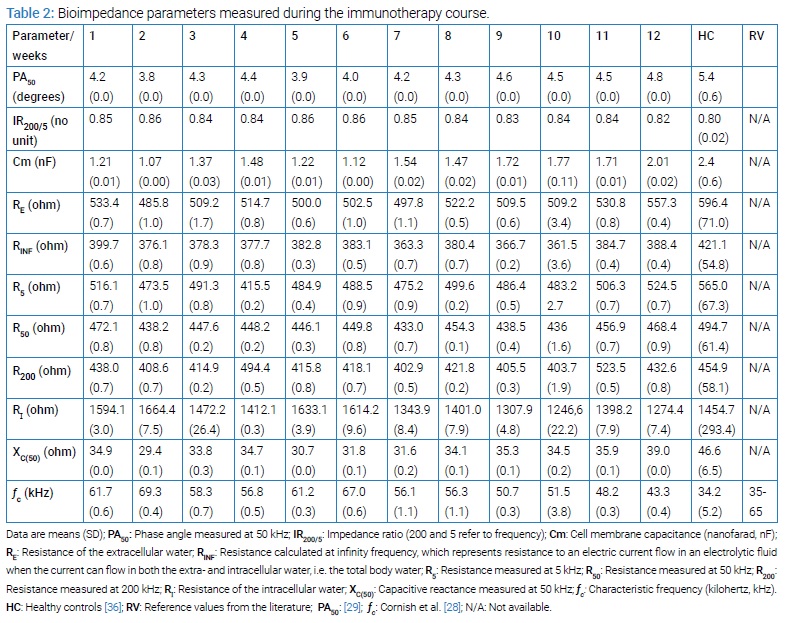
The trajectories of bioimpedance parameters are presented in (Table 2). Data for the three key parameters of this report (PA50, IR200/5 and Cm) are summarized below and presented graphically in (Figure).
From week 1 to week 12, PA50 increased from 4.2 degrees to 4.8 degrees (Table 2), approaching the mean ± SD range for healthy controls (5.4 ± 0.6 degrees; Table 2 and Figure a), but with deviation from reference values (6.19 ± 0.97 degrees; Table 2) [29].
IR200/5 decreased from 0.85 to 0.82 (no unit), with a range of 0.82–0.86 (mean 0.84) (Table 2). These values approached healthy controls’ mean ± SD range (0.80 ± 0.02; Table 2 and Figure b).
Cm increased from 1.21 nF to 2.01 nF over the twelve weeks of treatment (Table 2), achieving the mean ± SD range of the healthy controls (2.4 ± 0.6 nF; Table 2 and Figure c).
Discussion
This case report shows the clinical potential of using raw bioimpedance parameters in an NSCLC-patient undergoing a 12-week immunotherapy course. Such data may be used as physiological indices of hydration status (PA50, IR200/5) and cellular health (PA50, Cm), especially in cancer patients undergoing a long-term course of treatment. In addition, the data may prove to be important in the overall assessment and treatment of cancer patients. Furthermore, bioimpedance can be useful as an early biomarker of drug resistance, as seen in cancer therapy [30]. Data were collected from a whole-body BIA approach, with electrodes placed on hand and foot, where measurements represent average values of all cells along the conductive path (wrist to ankle). During the immunotherapy course, the patient experienced a general improvement in well-being supported by blood tests within normal ranges, CT scans that showed that the cancer was in rest or even diminished, and bioimpedance data approaching or reaching normal ranges. Among the bioimpedance parameters analyzed, changes in PA50 and Cm during the immunotherapy course were more marked than IR200/5. This may be explained by the fact that IR200/5 is a fully resistive parameter, i.e., related specifically to body water changes rather than cell structure and function. During treatment, the values of all three parameters approached the reference ranges from healthy controls or the literature. This suggests that the cancer patient initially experienced imbalances in hydration status and cellular functioning, but that treatment ameliorated these adverse changes. Imbalances in cellular functioning may be supported by previous findings showing that cancer cells have different electrical properties than the normal tissues surrounding them [31]. While BIA has been used previously in cancer patients, most studies have focused on using prediction equations to predict FFM and FM [32]. The novelty of the present case report is the use of raw bioimpedance parameters in a cancer patient undergoing a long-term cancer treatment course, parameters that are free of the assumptions underpinning the prediction of FFM and FM [32]. In practice, electrical parameters reduce the measurement uncertainty of prediction equations and remove the requirement that the equations fit the examined patient group. Furthermore, tissue electrical properties (R, XC, and PA) measured by BIA are more predictive than body-weight loss of prognosis in lung cancer patients [33].
A disadvantage of the present approach is that a BIS or multi-frequency BIA (MFBIA) device is required for measuring IR200/5 and Cm, while single-frequency BIA (SFBIA) devices operating at a fixed frequency of 50 kHz are in more common clinical use [23,26]. Furthermore, such devices only allow the calculation of PA50 (most often denoted “PA,” the 50 kHz frequency being implicit).
To confirm the results of this case report, there is a need for a study with a larger number of cancer patients. Furthermore, to build upon the findings presented in this case report, a relevant study would evaluate changes in PA50, IR200/5, and Cm in malnourished cancer patients undergoing various nutritional interventions since malnutrition is a major problem in this patient group [34]. In addition, there are few reference values, stratified by age and sex, available in the literature for the impedance parameters used here, which is why data from healthy controls were included. Therefore, future studies should focus on preparing reference values so it becomes possible for clinicians to interpret measurements performed on cancer patients or other clinical patients. Finally, it should be emphasized that standardization of BIA measurements is important to ensure data quality and clinical acceptance of the technique [10,35].
Conclusion
This case report demonstrates the potential of BIA and raw bioimpedance parameters to be biomarkers of changes in hydration status and cell function in cancer patients. Monitoring changes in these parameters may provide oncologists with important information about fluid balance and cell state changes during long-term cancer treatments, e.g., chemotherapy and immunotherapy. However, it is important to highlight that bioimpedance can only provide indications on some aspects of the quality of life of the cancer patient (e.g., the healthiness of the tissue), not the efficacy of the cancer treatment per se (e.g., whether metastases remain). At present, in routine practice, such information is typically inferred from the change in body-weight or BMI. Raw bioimpedance parameters are free of the assumptions underlying their use in prediction equations for body composition. Reference norms stratified by age and sex across the lifespan are required to increase clinical value and interpretation.
Acknowledgments
Thanks to the patient for participating in this case report, and for contributing to further understanding of the bioimpedance technique and the use of raw bioimpedance parameters in cancer patients.
Funding: No funding was received for this work.
Conflict of Interest
Author Leigh C Ward provides consultancy services to ImpediMed Ltd. ImpediMed Ltd had no involvement in the preparation of this manuscript. Other authors have no conflicts of interest to declare concerning this work.
References
- Barta JA, Powell CA, Wisnivesky JP. Global Epidemiology of Lung Cancer. Ann Glob Health. 2019;85(1):8.
- Brown JC, Cespedes Feliciano EM, Caan BJ. The evolution of body composition in oncology—epidemiology, clinical trials, and the future of patient care: facts and numbers. J Cachexia Sarcopenia Muscle. 2018;9(7):1200–1208.
- Cushen SJ, Power DG, Ryan AM. Nutrition assessment in oncology: top clin nutr. 2015;30(1):103–119.
- Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12(5):489–495.
- Prado CMM, Baracos VE, McCargar LJ, Reiman T, Mourtzakis M, Tonkin K, et al. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res Off J Am Assoc Cancer Res. 2009;15(8):2920–2926.
- Prado CMM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9(7):629–635.
- Tan BHL, Birdsell LA, Martin L, Baracos VE, Fearon KCH. Sarcopenia in an overweight or obese patient is an adverse prognostic factor in pancreatic cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2009;15(22):6973–6979.
- Prado CMM, Siervo M, Mire E, Heymsfield SB, Stephan BCM, Broyles S, et al. A population-based approach to define body-composition phenotypes. Am J Clin Nutr. 2014;99(6):1369–1377.
- Thibault R, Pichard C. The evaluation of body composition: a useful tool for clinical practice. Ann Nutr Metab. 2012;60(1):6–16.
- Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Gómez JM, et al. Bioelectrical impedance analysis-part II: utilization in clinical practice. Clin Nutr Edinb Scotl. 2004;23(6):1430–1453.
- Nielsen BM, Dencker M, Ward L, Linden C, Thorsson O, Karlsson MK, et al. Prediction of fat-free body mass from bioelectrical impedance among 9- to 11-year-old Swedish children. Diabetes Obes Metab. 2007;9(4):521–539.
- Mulasi U, Kuchnia AJ, Cole AJ, Earthman CP. Bioimpedance at the bedside: current applications, limitations, and opportunities. Nutr Clin Pract. 2015;30(2):180–193.
- Kuchnia AJ, Teigen LM, Cole AJ, Mulasi U, Gonzalez MC, Heymsfield SB, et al. Phase angle and impedance ratio: reference cut-points from the united states national health and nutrition examination survey 1999–2004 from bioimpedance spectroscopy data. JPEN J Parenter Enteral Nutr. 2017;41(8):1310–1315.
- Brantlov S, Jødal L, Andersen RF, Lange A, Rittig S, Ward LC. Bioimpedance resistance indices and cell membrane capacitance used to assess disease status and cell membrane integrity in children with nephrotic syndrome. Scientific World Journal. 2019;2019:4274856.
- Brantlov S, Jødal L, Andersen RF, Lange A, Rittig S, Ward LC. An evaluation of phase angle, bioelectrical impedance vector analysis and impedance ratio for the assessment of disease status in children with nephrotic syndrome. BMC Nephrol. 2019;20(1):331.
- Norman K, Stobäus N, Pirlich M, Bosy-Westphal A. Bioelectrical phase angle and impedance vector analysis--clinical relevance and applicability of impedance parameters. Clin Nutr Edinb Scotl. 2012;31(6):854–861.
- Selberg O, Selberg D. Norms and correlates of bioimpedance phase angle in healthy human subjects, hospitalized patients, and patients with liver cirrhosis. Eur J Appl Physiol. 2002;86(6):509–516.
- Baumgartner RN, Chumlea WC, Roche AF. Bioelectric impedance phase angle and body composition. Am J Clin Nutr. 1988;48(1):16–23.
- Lukaski HC, Kyle UG, Kondrup J. Assessment of adult malnutrition and prognosis with bioelectrical impedance analysis: phase angle and impedance ratio. Curr Opin Clin Nutr Metab Care. 2017;20(5):330–339.
- Thibault R, Makhlouf AM, Mulliez A, Gonzalez MC, Kekstas G, Kozjek NR, et al. Fat-free mass at admission predicts 28-day mortality in intensive care unit patients: the international prospective observational study Phase Angle Project. Intensive Care Med. 2016;42(9):1445–1453.
- Knudsen NN, Kjærulff TM, Ward LC, Sæbye D, Holst C, Heitmann BL. Body water distribution and risk of cardiovascular morbidity and mortality in a healthy population: a prospective cohort study. PloS One. 2014;9(2):e87466.
- Demirci C, Aşcı G, Demirci MS, Özkahya M, Töz H, Duman S, et al. Impedance ratio: a novel marker and a powerful predictor of mortality in hemodialysis patients. Int Urol Nephrol. 2016;48(7):1155–1162.
- Matthie JR. Bioimpedance measurements of human body composition: critical analysis and outlook. Expert Rev Med Devices. 2008;5(2):239–261.
- Cornish BH, Lingwood BE, Ward LC. Can bioimpedance spectroscopy (BIS) tell us about the form of lymphoedema? In 13th International Conference on Electrical Bioimpedance and the 8th Conference on Electrical Impedance Tomography: Springer, Berlin, Heidelberg; 2007. p. 795–798.
- Małecka-Massalska T, Mlak R, Smoleń A, Brzozowska A, Surtel W, Morshed K. Capacitance of membrane as a prognostic indicator of survival in head and neck cancer. PloS One. 2016;11(11):e0165809.
- Brantlov S, Jødal L, Lange A, Rittig S, Ward LC. Standardisation of bioelectrical impedance analysis for the estimation of body composition in healthy paediatric populations: a systematic review. J Med Eng Technol. 2017;41(6):460–479.
- Thomas BJ, Cornish BH, Ward LC. Bioelectrical impedance analysis for measurement of body fluid volumes: a review. J Clin Eng. 1992;17(6):505–510.
- Cornish BH, Ward LC, Thomas BJ, Jebb SA, Elia M. Evaluation of multiple frequency bioelectrical impedance and Cole-Cole analysis for the assessment of body water volumes in healthy humans. Eur J Clin Nutr. 1996;50(3):159–164.
- Barbosa-Silva MCG, Barros AJD, Wang J, Heymsfield SB, Pierson RN Jr. Bioelectrical impedance analysis: population reference values for phase angle by age and sex. Am J Clin Nutr. 2005;82(1):49–52.
- Fuentes-Vélez S, Fagoonee S, Sanginario A, Gallo V, Riganti C, Pizzi M, et al. Impedance-based drug-resistance characterization of colon cancer cells through real-time cell culture monitoring. Talanta. 2021;222:121441.
- Blad B, Baldetorp B. Impedance spectra of tumour tissue in comparison with normal tissue; a possible clinical application for electrical impedance tomography. Physiol Meas. 1996;17 Suppl 4A:A105–A115.
- Ward LC. Bioelectrical impedance analysis for body composition assessment: reflections on accuracy, clinical utility, and standardisation. Eur J Clin Nutr. 2019;73(2):194–199.
- Toso S, Piccoli A, Gusella M, Menon D, Bononi A, Crepaldi G, et al. Altered tissue electric properties in lung cancer patients as detected by bioelectric impedance vector analysis. Nutrition. 2000;16(2):120–124.
- Arends J, Baracos V, Bertz H, Bozzetti F, Calder PC, Deutz NEP, et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin Nutr. 2017;36(5):1187–1196.
- Brantlov S, Ward LC, Jødal L, Rittig S, Lange A. Critical factors and their impact on bioelectrical impedance analysis in children: a review. J Med Eng Technol. 2017;41(1):22–35.
- Pedersen CB. The danish civil registration system. Scand J Public Health. 2011;39(7 Suppl):22–25.
Keywords
Bioimpedance; Electrical impedance; Cancer; Cell; Hydration
Cite this article
Brantlov S, Jødal L, Heitmann BL, Ward LC. Clinical value of bioimpedance during long-term cancer therapy. Clin Case Rep J. 2021;2(5):1–6.
Copyright
© 2021 Steven Brantlov. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-4.0).



