An Electrophysiological Investigations of the Human Triceps Surae Muscle after a Simulated Microgravity Environment
* Yuri A Koryak;
-
* Yuri A Koryak: Department of Sensory-Motor Physiology and Countermeasures, Institute of Biomedical Problems of the Russian Academy of Sciences, Moscow, Russia.
-
Feb 22, 2022 |
-
Volume: 3 |
-
Issue: 2 |
-
Views: 1908 |
-
Downloads: 2361 |
Abstract
This paper compares the effects of 7-day “dry” water immersion (DI) and intermittent muscle contractions on electrical and mechanical failure during muscle fatigue in the human Triceps Surae (TS) muscle electrically stimulated at 50 impulses s-1 via its motor nerve. 1-s intervals separated intermittent contractions of 60-s duration for the identical duration of tension development. The 60-s intermittent contractions decreased tetanic force to 57% (p < 0.05) of initial values, but force reduction was not significantly different in the two fatigue tests: the fatigue index was (36.2 ± 5.4)% vs. (38.6 ± 2.8)%, respectively (p > 0.05). While identical force reduction was present in the two fatigue tests, it would appear that concomitant electrical failure was considerably different. This electromechanical dissociation would suggest that slowing conduction along nerve and muscle membranes did not explain the observed mechanical failure. It is suggested that intracellular processes played a major role in contractile failure during intermittent contractions after muscle disuse.
Introduction
It is well known that mechanical force output decreases gradually during muscle contraction. This “fatigue” phenomenon is probably one of the most intriguing observations associated with contractile activity. Human muscle fatigue studies have been performed under a variety of experimental conditions and, in many cases, the concept of fatigue is applied to assessing deterioration of muscle performance, i.e., “the failure point” at which the muscle is no longer able to sustain the required force or work output level [1] or as “an inability to maintain the required or expected force” [2]. However, despite numerous investigations, the etiology of muscle fatigue remains unknown [3].
When a muscle is activated by electrical stimulation at a constant frequency, fatigue is defined as decreased tension. Few easily measurable, objective physiological indices of fatigue exist. These maximally stimulated contractions serve as an index of muscle contractility and are independent of the central drive [4,5]. Changes in the surface electromyogram (EMG) have been used extensively as indices of fatigue [6–8]. However, the relationship between the EMG and fatigue remains unclear, and EMG changes often precede muscle fatigue [7], and interpretations of the EMG signal and fatigue are made difficult by the complexity of the EMG signal. Therefore, the exact relationship between the EMG signal and muscle fatigue has not been defined despite extensive use. The EMG recorded from a muscle during volitional activation is a summation and interference of the motor unit action potentials (AP) from all the active motor units [9], and the characteristics of the EMG depend on the firing patterns of the motor units as well as on the configuration of the motor units Apps [8].
The physiological data within the muscle show time-dependent changes during the time course of muscle fatigue development. Such changes are related to hydrogen ion and metabolite accumulation [10–12] and sodium and potassium ion concentration shifts [5]. These changes would, in turn, affect the muscle excitation-contraction coupling E-C coupling) including the muscle membrane properties, and muscle AP propagation, leading to EMG manifestations of muscle fatigue distinct from mechanical manifestations [13,14]. There is possibly no single cause, and fatigue may occur at several sites independently.
The physiological and biochemical properties of limb skeletal muscle have been shown to adapt to various experimental conditions. Among these is the microgravity encountered with spaceflight [15,16]. Foremost among these changes is a reduction in the force-generating capacity [11,12], which is presumably a direct result of a decrease in fiber number [17] and diameter [17,18]. A decline in fiber number or diameter may coincide with the loss of active cross bridges, as suggested by the decreased tetanic tension after disuse [11,12]. Furthermore, disuse has been shown to reduce the functional capacity of the sarcoplasmic reticulum [19], and this may in part explain the observed decrease in the rate of tension development and decline [11,12]. These changes suggest a spaceflight-induced reduction in muscle work capacity. Thus attention has been focused on the relationship between disuse and changes in intracellular content, which contribute to the control of muscle contraction kinetics. The suggestion is that changes in muscle membrane ionic processes might contribute to the observed reduction of contraction kinetics after disuse [20]. The present work examines in humans the effects of a series of 60-s intermittent contractions separated by intervals series of 1-s on mechanical and electrical failure in the TS after a 7-day DI. Mechanical and electrical parameters were recorded during electrical stimulation of the muscle motor nerve to dissociate peripheral sites from the central level [21].
Materials and Methods
Prior to the experiment, details and possible risks of the protocols were explained to the subjects, and written informed consent was obtained from each of them. None of them had а habit of exercising оn а regular basis. The experimental protocol was approved bу the Russian National Committee on Bioethics of the Russian Academy of Sciences and complied with the principles outlined in the Declaration of Helsinki.
Subjects: Experiments were performed on six healthy male subject-volunteers. The groups’ mean ages, body heights, and masses were 22.7 years ± 3.5 years, 1.76 m ± 0.3 m, and 66.4 kg ± 2.3 kg, respectively. All subjects were habitually active and had a lean body composition. Selection of subjects was based on a screening evaluation that consisted of a detailed medical history, physical examination, complete blood count, urinalysis, resting and cycle ergometer electrocardiogram, and a panel of blood chemistry analysis, which included fasting blood glucose, blood urea nitrogen, creatinine, lactic dehydrogenase, bilirubin, uric acid, and cholesterol. All of the subjects were habitually active and had a lean body composition. No subject was taking medication at the study, and all were nonsmokers.
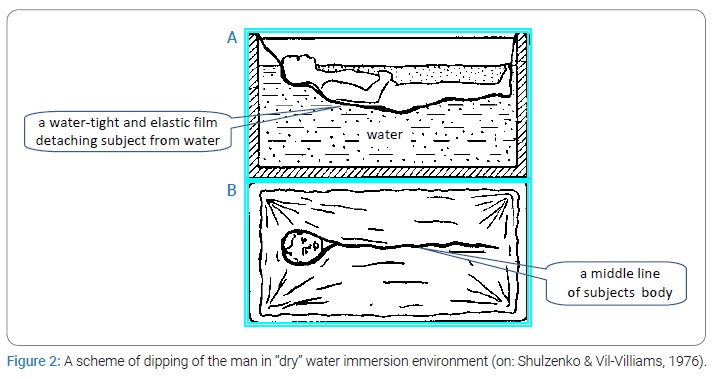
“Dry” water immersion (DI): DI was used to simulate a microgravity effect [22]. Each subject was positioned horizontally in a special bath on fabric film that separated him from the water (Figure 1). During immersion, the subjects remained in a horizontal position (an angle which makes the body and horizontal line, e.g., 5º head-up position) continuously for all activities, including excretory function and eating. The duration of the DI was 7-days. The water temperature was constant (33.4°C) and maintained automatically at this level throughout the experiment. The subjects were kept under medical observation. The functional properties of the neuromuscular system were evaluated twice: 8 days–10 days before the beginning of DI and after it ended. The test protocol was identical for both pre-and post-DI tests.
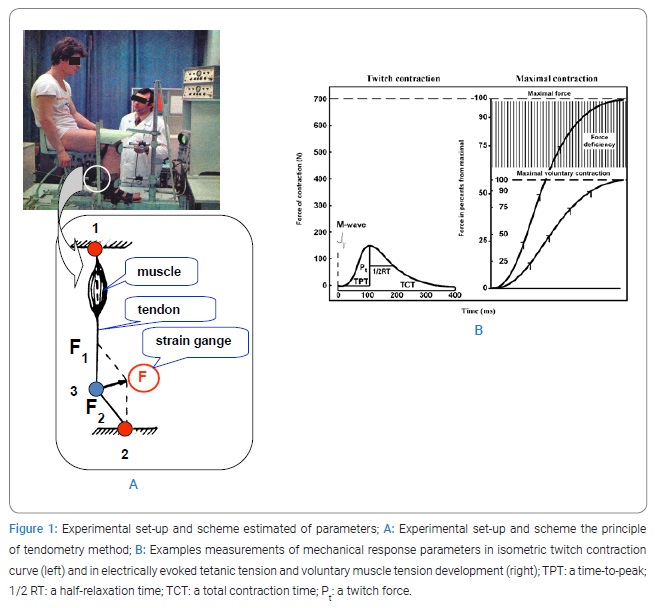
The testing procedure and measurement: The mechanical responses of the TS were recorded by tendometry, which made it possible to measure the force of a single muscle contraction by the degree of tension change in the distal muscle-tendon [23]. Measurement of muscle tension using a strain-gauge transducer is based on the physical law of the resolution of forces according to the parallelogram principle (Figure 2A). If a strain-gauge the transducer is pressed to the tendon; the transducer causes it to bend at an angle. The force (F1) directed along the muscle axis to the proximal point of attaching and originates during the muscle contraction is oppositely directed and equal to the force (F2) directed to the distal point of the tendon attachment. F1, which is directed across the tendon, operates at the point of the transducer and tendon contraction. If the angle at which the tendon bends is constant, the force (F) recorded by the strain-gauge dynamometer is proportional to F1 (or F2). A rigid dynamometer is needed to record the muscle force using a strain-gauge transducer because any deformation under tendon pressure will change the transducer’s position and alter the tendon angle. A steel dynamometer ring was used in our transducer.
The endometrial sensor was a steel ring of dynamometer with a saddle-shaped special block attached to its surface to support the tendon. The pressure between the endometrial sensor and the tendon was constant for all the subjects and amounted to 5 kg. A special set-up was designed to ensure standardization of position and fixation of the limb during the assessment, as shown in (Figure 2A). The apparatus maintained the thigh and lower leg in a standardized position (knee joint angle between the tibia and sole of the foot at 90 degrees). The position of the seat was adjusted to the individual and then firmly secured. A rigid leg fixation ensured isometric conditions for muscle contraction. The stimulation was performed by supramaximal voltage rectangular wave pulses of 1 ms duration and with the frequency of 150 Hz [23] and 50 Hz [24].
The isometric twitch and tetanic contractions of the TS muscle were induced by electrical stimulation of the tibial nerve using supramaximal rectangular pulses of 1-ms duration with a frequency of 150 Hz for the tetanic contractions [23]. To stimulate the muscle, the active electrode (cathode, 1 cm in diameter) was located in the popliteal fossa, which is the place of the lowest resistance, and the anode (a 6 cm x 4 cm in size) was positioned on the lower third of the front of the thigh. The maximal isometric peak twitch force (Pt) was measured from the tendogram of the TS muscle isometric twitch response to a single electrical stimulus applied to the tibial nerve (Figure 2B). In addition, the time from the moment of stimulation to peak twitch (TPT), the time from contraction peak-to-half relaxation time (1/2 RT), and total contraction time (TCT) - the time from moment of stimulation to the total muscle relaxation - were calculated from the tendogram of the isometric twitch (Figure 2B).
The maximal voluntary contraction (MVC) was measured from the tendogram of an isometric voluntary contraction performed after the subject had been instructed to contract maximally (Figure 2B). The MVC was determined from three 3–4 s duration contractions separated by 3 min, the largest being considered maximal.
The subjects were verbally encouraged during the contractions, and visual feedback was provided. The maximal strength of the contraction (P0) evoked in response to tetanic electrical stimulation of the tibial nerve innervating the TS muscle at a frequency of 150 Hz was measured from the tendogram as has been described by Koryak [23,24]. The difference between P0 and MVC expressed as a percentage of the P0 and referred to as force deficiency (Pd, Figure 2B) was also calculated [23].
The rate of development of increased muscle tension was calculated from the tendogram by the isometric voluntary contraction after the instruction to exert the fastest and greatest tension using a relative scale, i.e., the time of reach 25%, 50%, 75%, 90% of maximal tension [23,24]. Similarly, measurements were made for the rate of rising the evoked contraction in response to electrical stimulation of the nerve with a frequency of 150 Hz [23].
The maximal rates of voluntary (dPvc/dt), and twitch (dPt/dt), and tetanic development (dPec/dt), and tetanic relaxation (dPec/dt), were obtained by differentiation of the analogue signal. At the end of the tests, after full rest (4 min–5 min), the fatiguability of the skeletal muscle was evaluated. The contractile features of the TS muscle were studied during a standard series of 60 1-s electrically evoked (50 Hz) isometric contractions separated by 1-s intervals as has been described [7]. The stimulation frequency was 50 Hz during fatigue tests because it is within the physiological frequency range of activation of muscle cells during the initial part of strong voluntary contractions [8] and is the value that gives the maximal isometric tetanic contraction in response to tetanic electrical stimulation of the nerve innervating the TS muscle [23].
During the fatigue tests, recording of electrical muscle activity, called electromyography (EMG) or surface action potential (SAP) in the test, was achieved by means of Ag-AgCl surface electrodes (8 mm diameter). The two recording electrodes were placed longitudinally over the soleus muscle belly with their centers 25 mm apart. The inter-electrode impedance was less than 5 kΩ. The large grounding electrode (7.5 cm x 6.5 cm) was located in the proximal portion of the leg between the pick-up and stimulating electrodes. Recording of the mechanical (tendometry) and electrical (SAP) responses of the skeletal muscle during intermittent contractions was made during 1-s after the start “of” tetanization of the motor nerve and then for short periods (about 0.2 s) at the end of each subsequent contraction.
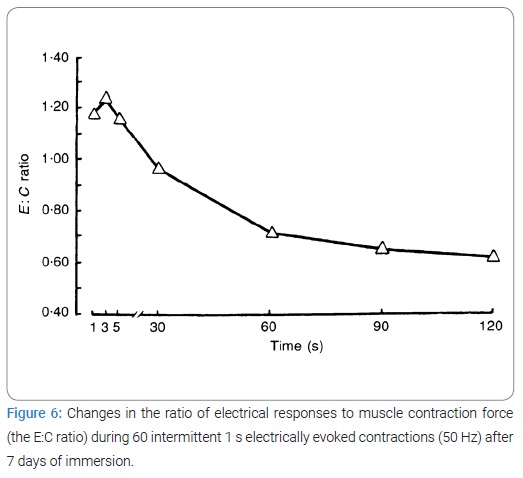
The EMG was analyzed from the amplitude of the electrical responses (M-waves, peak-to-peak amplitude of SAP [15,18]) as well as the amplitude, duration, and area of the first phase of the SAP at the end of 1 s, 3 s, 5 s, 61 s and 121 s of rhythmic muscle contraction. To determine the relative extent of changes in contractile (C) and electrical (E) function, the E:C ratio was calculated where E was is the amplitude of the M-wave and C the mechanical response. The E:C ratio was calculated at the end of contractions at 1 s, 3 s, 5 s, 61 s and 121 s from the start of electrical tetanization. The experiment results were simultaneously recorded on magnetic tape, and the SAP was also recorded on a storage oscilloscope.
The fatiguability of the TS muscle was calculated as the fatigue index, being the mean loss of force of the last five contractions, expressed as a percentage of the mean value of the first five contractions [7].
Force and EMG recordings: The mechanical responses of the TS were recorded by tendometry, which made it possible to measure a single muscle contraction force by the degree of tension change in the distal muscle tendon. To ensure standardization of position and fixation of the limb during the assessment, a special set-up was designed [25,26]. The apparatus maintained the thigh and lower leg in a standardized position (knee joint angle between tibia and sole of the foot of 90 deg).
Recording of electrical muscle activity called electromyography (EMG) or surface action potential (SAP) in the test was achieved through Ag-AgCl surface electrodes (8 mm diameter). The two recording electrodes were placed longitudinally over the soleus muscle belly, their centers 25 mm apart. The inter-electrode impedance was less than 5 kΩ. The earth electrode (7.5 cm x 6.5 cm) was fastened on the skin at the shank. Recording of the mechanical and electrical responses of the skeletal muscle during intermittent contractions was made during the first 1-s tetanus and then for a short period (about 0.2 s) at the end of each subsequent contraction.
Measurements and statistics: Experimental EMG- and force-signals were recorded on magnetic tape, and the SAP supplementally was displayed on a storage oscilloscope. We measured the mechanical twitch- and tetanic-tension development (Pt and P0, respectively) as well as twitch time-to-peak (TPT) and time to half relaxation (1/2 RT). The maximum rates of twitch tension development (dPt/dt) were obtained by differentiation of the analog signal.
The fatiguability of the TS was calculated from the fatigue index (FI) - the percentage ratio of an average force of the five last contractions to that of five first contractions [27]. The EMG was analyzed from the amplitude of the electrical responses - M-waves [peak-to-peak amplitude of SAP [28,29]] as well as the amplitude (V), duration (D), area (A) of the first phase of the SAP [30] at the end of the 1 s, 3 s, 5 s, and 121 s of the rhythmic muscular contraction. To determine the relative extent of change in contractile (C) and electrical (E) M-waves as a result of fatiguability, the E/C ratio was calculated where E was the ratio of the amplitude of electrical post-immersion M-wave to the corresponding pre-immersion M-wave and C was the ratio of post-immersion to corresponding pre-immersion mechanical response of TS. The E/C ratio was determined from the indices of E and C at the end of 1 s, 3 s, 5 s, and 121 s from start electrical tetanization.
Statistics: All values reported are the mean ± SE. The Student is paired t-test, and unpaired Wilcoxon test determined differences between group means using the 0.05 level of confidence. In addition, the percentage changes for pre-immersion and post-immersion were calculated.
Results
Immersion and strength: The effects of DI on the MVC are illustrated in (Figure 3) (left panel). A decrease in MVC was consistently observed in all subjects by a mean of 18.9% (p < 0.01). The values of Pt and P0 were not statistically significantly different to the control value [pre 125.6 ± 13.7) N compared to post-immersion 139.3 ± 18.6) N and 643.5 ± 34.3) N compared to post-immersion 590.5 ± 56.8) N, respectively].
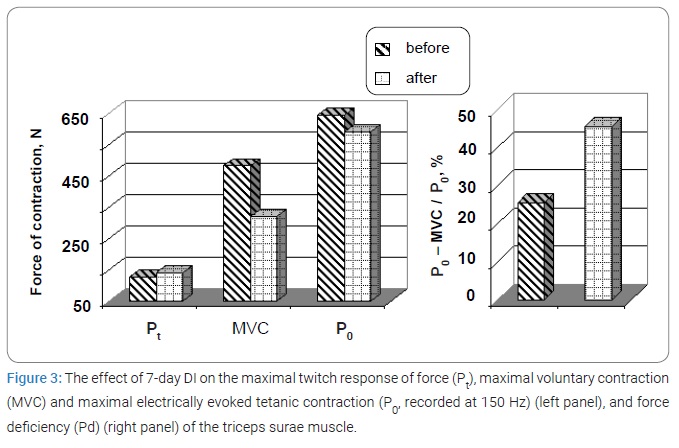
The Pd increased significantly by a mean of 44.1% [pre 25.4 ± 3.1) % compared to post-immersion 45.4 ± 4.9) %, respectively; p < 0.001] (Figure 3, right panel).
Fatigue and tetanic force: The effects of 7-day DI on the electrically evoked intermittent contractions stimulated at 50 Hz are illustrated in (Figure 5A). In this example, tetanic force decreased gradually to about 57% of its initial value (recorded from the same muscle at 50 Hz). There were no significant differences between the measurements made before and after immersion: the fatigue indices were 36.2% ± 5.4% vs. 38.6% ± 2.8%, respectively, (p > 0.05). In addition to the loss of force, there were changes in the kinetics of contraction. The time course of the contractile activity of the muscle revealed a number of phases characterized by changes in the speed of contraction. There was an increase in speed compared to the initial value at 30 s–31 s (17%–18%) with a subsequent relative slowing-down at 60 s–61 s (8%–9%) followed by a slight rise at 90 s–91 s (11%–12%) with a slowing-down in the last phase (3%–7%). (Figure 5) reflects the dynamics of a decrease in the force of a muscle. High force isometric contractions interfere with intramuscular blood flow [31], and the different phases of the fatigue curve could be associated with alterations in blood flow as muscle force production changes. It is of particular interest that there were no changes in the fatigue curves of the muscle after disuse.
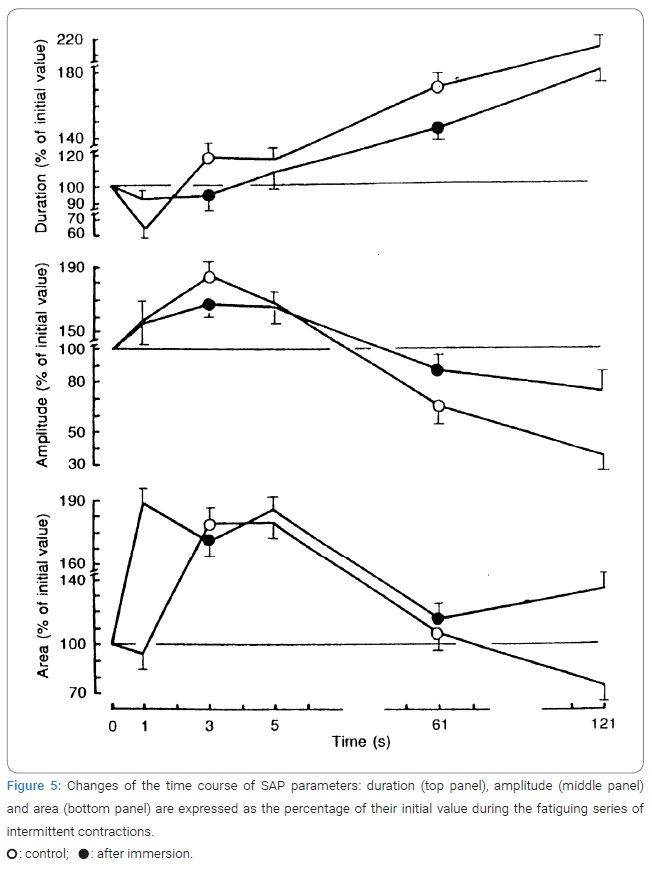
Fatigue and electrical processes: The reduction of muscle force output could be explained by a decrease in electrical activity. (Figure 4) shows that in both tests, SAP duration decreased significantly during the first 3 s and the corresponding amplitude and area increased. After that, the duration of the negative SAP phase increased throughout the test, whereas the time courses of SAP amplitude and area were quite different.
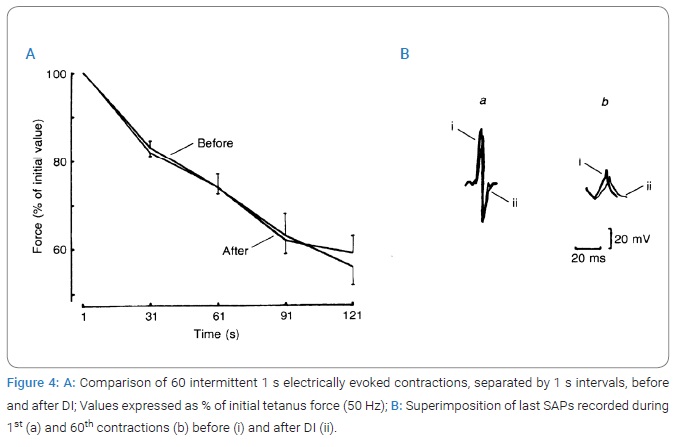
Their initial increases observed during the first 150 and 250 responses, respectively, were followed not only by a drop to the initial level but also by the inversion of the measured parameters. At the same time, the arrangement of the curves has engaged attention: the increase and/or decrease of the electrical M-waves were greater after DI when compared to the control. Thus, after disuse, with fatiguability, both the force of electrically induced contraction and the electrical M-waves (or SAP) were reduced significantly with fatigue. However, after eliminating the gravitational loading, there were no differences in the dynamics of the decrease in the TS muscle contraction during an intermittent electrically evoked test to induce fatigue. However, there were significant differences in the dynamics of the changes in electrical responses. The relative extent of the decline in either of these two parameters could be determined from the change in the relationship of the electrical M-wave to the mechanical response of the TS muscle (the E:C ratio).
After a 7-day immersion, the amplitude of the SAP first phase was reduced by 77.3% ± 10.7%, whereas its duration was increased by 70.2% ± 3.6% during 121 s intermittent contraction in comparison with the first 1 s, whereas in control muscles, the amplitude was reduced by only 45.0% ± 3.1% and duration was increased by only 52.2% ± 12.2% during an identical fatigue test (Figure 4B).
Analysis of the dynamics of the E:C ratio change revealed a significant difference (Figure 5). As shown by this ratio, during muscle work of the same intensity, there may have been different changes in the status of the contractile and electrogenic elements of the peripheral neuromuscular system. The ratio also suggests that after immersion during the initial 5 s of the test contraction, there was a relatively more marked decrease in the contraction force than the electrical M-waves. With the increasing duration of the work performed, there was a relatively more marked decrease in the electrical muscle responses.
Fatigue and E-C coupling: E-C coupling was examined by delivering a single supramaximal stimulus of short duration (1 ms) and studying the resulting response, both of the SAP and the twitch force.
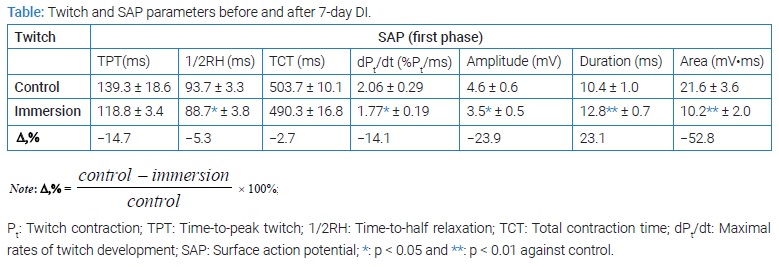
(Table and Figure 6) compares E-C coupling in extreme situations before and after immersion. A study of mechanical twitch indicates that with constant Pt and TPT of muscle but maximum dPt/dt expressed as absolute values decreased significantly. The normalized value (% of Pt) dPt/dt decreased by 5.3% [pre 1.50 ± 0.08) % Pt/ms compared to post-spaceflight 1.42 ± 0.04) % Pt/ms; p < 0.05]. The 1/2RT and TCT reduced by 5.3% and 2.8%, respectively.
Interestingly, the comparison of these mechanical twitch showed no significant difference. The Pt/P0 ratio was reduced by 8.7%. The concomitantly recorded electrical properties indicated that the soleus muscle SAP was significantly changed after immersion (Table). The amplitude of SAP was decreased by 14.6% (p < 0.05), the duration was prolonged by 18.8% (p < 0.01), and the area was found to be decreased by 2.8% after DI. Interestingly, the comparison of these mechanical twitch shows no significant difference. This electromechanical dissociation suggests that not only failure of electrical propagation is present, but that some step beyond membrane processes is changed.
Discussion
Mechanical failure (fatigue) during contraction is probably one of the most intriguing physiological phenomena of muscle capacity. The present work is a contribution to the discussion of this problem. It examines the specific effects of disuse and the electrical and mechanical changes in intact human the TS, during intermittent contractions elicited of electrical stimulated its motor nerve [32]. Our experimental procedure excludes the possible effects of central (motor) nervous command during these fatigue tests (cf. Materials and Methods).
Our results show that:
- The rate of decrease in the force of muscle contraction during rhythmic fatigue stimulation does not vary in control and after disuse.
- On stimulation, the SAP showed a marked decline in amplitude and increase in duration, reflecting changes in the peripheral generation of the APs by the muscular fibers.
- A correlation between the electrical and mechanical responses of the muscle (ratio E/C) indicates that the specific role of fatigue of “electrogenic” and “contractile” elements of the neuromuscular system is changed during the development of peripheral fatigue.
Our findings (Figure 1A) do not reveal the difference in the reduction of the working capacity before and after disuse which is in good agreement with previously obtained data [33,34] and confirm Merton’s point of view [31] that peripheral mechanisms play an important role in a force reduction. Peripheral fatigue probably results from a deterioration in the excitability of the muscle fibers. Failure of propagation of action potentials may occur;
- Along the terminal branches of motor nerves [23].
- At the neuromuscular junction [23].
- Along the surface of muscle fibers [4,23] and along the T-tubules [35].
The blocking of the fiber APs during stimulation indicates excitation failure, but the location cannot be determined. Blocking of an SAP was invariably preceded by a major change in the amplitude and duration of the fiber AP.
An integral index of the state of the “electrogenic” element of the neuromuscular system can be the size (amplitude and area) of the recorded SAP. Comparison of electrical and mechanical failures during intermittent contractions is interesting because this comparison indicated that identical tetanic force reduction after and before disuse (Figure 1B) is associated with complex SAP change (Figure 2). This observation suggests that muscle intracellular processes must play a major role in the observed contractile failure.
Recorded SAP changes during fatigue indicate that different peripheral mechanisms may be involved. The comparison between control and disused muscle indicates that the failure of the electrical processes is different in the two fatigue tests. It appears from (Figure 2) that during the first 3 s of contraction, the SAP durations decrease in both tests while the corresponding amplitude and area by the SAP significantly increased. These data suggest that presynaptic and/or end-plate potentials are facilitated, that the propagation velocity of the AP increases along the muscle membranes [36], and that the dispersion between the fiber action potentials is reduced [37,38]. The subsequent increase in SAP duration and area, observed without any reduction in SAP amplitude, must be due mainly to the slowing of the conduction velocity along membranes of muscle fibers [4,39] so the SAP broadens in shape (Figure 2).
In the second half of the intermittent contractions, the reduction in SAP amplitude suggests that presynaptic and/or end-plate failure is now present in intermittent fatigue. This view is consistent with the finding that the SAP area, which was previously found to increase, now decreases, although the duration of the SAP keeps increasing throughout the test. It is also consistent with the previous observation that the rise time of the end-plate current decrease during disuse [40,41], although the decrease in contraction force is identical in both fatigue intermittent tests. This difference in behavior between nerve and muscle membranes is not surprising since their architectures are quite different, and recovery of control ionic concentrations must be slower in the muscle T (transverse) system compared with nerve membranes. The presence of a T (transverse) tubular system was suggested to play an important role in electrical muscle fatigue [24,42].
The study of muscle E-C coupling is achieved by comparing twitch SAP and tension development. Our results show that dPt/dt are reduced, but Pt was not significantly changed after disuse, whereas changes of corresponding SAP are considerably different. This electromechanical dissociation suggests not only failure of electrical propagation but that some step beyond membrane processes is changed and plays a preponderant role [5,31,35]. Changes in the configuration of AP have been associated with a depletion in extracellular [Na+], an increased [K+], and an increase in [H+] ion of muscle fibers [4,5,24]. In addition, these suggest that muscle energy metabolism can play an important role in regulating muscle membrane excitability. An additional factor of enhancing the electrolytes excretion can be the disuse significantly impairing the electrolytic homeostasis [43] and thus affording the reduction of muscle membrane excitability [2,4,5,7]. In our case, the surface EMG presented in (Figure 2) strongly supports this hypothesis.
As is evident from our findings, the reduction in the contraction force of the muscle during the intermittent fatigue test was similar in control and after disuse, suggesting, on the one hand, that developed fatigue cannot be explained by the changes in the contractile apparatus itself as a result of acting factor. On the other, one of the components (if not the single cause) of developing peripheral fatigue can be the disorders in the electrogenic element of the neuromuscular system [44]. Comparing the changes in mechanical response with a corresponding alteration of electrical response (ratio E/C) enables one to determine the specific role of the electrogenic and contractile elements in the development of peripheral fatigue. Analysis of the ratio E/C before and after disuse indicated that there occurs the different dynamics of changes in the state of contractile and electrical elements of the peripheral neuromuscular system. These results are not contradictory to the above discussions because an interpretation of the ratio E/C suggests a linear relationship between mechanical responses of muscle. However, the relationship between the EMG and fatigue remains unclear [7], especially when developing fatigue can be impaired. During contraction triggered by electrical stimulation of the motor nerve, the decrease in the contractile force of the muscle cannot be compensated by a modulation of the motor unit firing frequency [45]. The study of electrically evoked fatigue shows that the decrease in force observed during intermittent contractions is not different in control and disused muscles and indicates that fatigue is now not larger after DI. Besides, the normal action potential of muscle fiber is several times higher than the threshold value required for activation of the contractile apparatus [46]. Due to this, even reduced APs can trigger the normal contraction of muscle fibers.
Conclusion
During the short exercise duration, the decreased muscle force appears to result essentially from peripheral neuromuscular changes. The study of electrically evoked contractions contributes to understanding the underlying mechanisms of peripheral fatigue in the neuromuscular system since it avoids the complications resulting from changes in the motor unit firing frequency.
Mechanical failure during intermittent muscle contraction involves peripheral sites and mechanisms associated with membrane and intracellular processes. The comparison of peripheral electrical and mechanical changes during intermittent electrically triggered contractions in both contractions indicates that the slowing of AP conduction along nerve and muscle membranes does not explain the observed mechanical failure.
Comparing the electrical and mechanical responses recorded in control and disuses muscle supports the proposition that electrical changes do not closely control mechanical failure. Thus among the peripheral neuromuscular changes observed during short-duration exercises, muscle intracellular processes must play the dominant role concerning the observed force decrease during fatigue after disuse.
Acknowledgments
The author wishes to express his appreciation to all people who contributed to the successful performance of the experiment. He is incredibly grateful to Mr. Vladimir Gratchev for the work’s technical design and very thankful to Mr. Yuri Voronkov, Manager of the Department of Medical Clinic, and Mrs. Elena Dobrokvashena, physician of the Department of Medical Clinic, monitor the subjects.
The author thanks two anonymous reviewers for their insightful critiques of the manuscript, and he also gratefully acknowledges the six subjects that endured 7-d confinement.
Funding
This work was financially supported by Ministry of Education and Science of the Russian Federation (Grant reference 075-1502020-919).
Conflict of Interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Informed consent was obtained for this publication.
References
- Moritani T, Nagata A, de Vries H, Muro M. Critical power as a measure of physical work capacity and anaerobic threshold. Ergonomics. 1981;24(5):339–350.
- Edwards RH. Human muscle function and fatigue. In: Porter R, Whelan J, editors. Human Fatigue: Physiological Mechanisms. London: Pitman Medical; 1981.p. 1–18.
- Fitts RH, Courtright JB, Kim DH, Witzmann FA. Muscle fatigue with prolonged exercise: contractile and biochemical alterations. Am J Physiol. 1982;242(1):C65–C73.
- Bigland-Ritchie B, Jones DA, Woods JJ. Excitation frequency and muscle fatigue: electrical responses during human voluntary and stimulated contractions. Exp Neurol. 1979;64(2):414–427.
- Jones DA, Bigland-Ritchie B, Edwards RH. Excitation frequency and muscle fatigue: mechanical responses during voluntary and stimulated contractions. Exp Neurol. 1979;64(2):401–413.
- Edwards RG, Lippold OC. The relation between force and integrated electrical activity in fatigued muscle. J Physiol. 1956;132(3):677–681.
- Lindstrom L, Kadefors R, Petersen I. An electromyographic index for localized muscle fatigue. J Appl Physiol Respir Environ Exerc Physiol. 1977;43(4):750–754.
- Lindstrom J, Magnusson RT. Interpretation of myoelectric power spectra: a model and its applications. Proc IEEE. 1977;65(5):653–662.
- De Luca CJ. Physiology and mathematics of myoelectric signals. IEEE Trans Biomed Eng. 1979;26(6):313–325.
- Wilkie DR. Muscular fatigue: effects of hydrogen ions and inorganic phosphate. Federation Proc. 1986;45(13):2921–2923.
- Witzmann FA, Kim DH, Fitts RH. Recovery time course in contractile function of fast and slow skeletal muscle after hindlimb immobilization. J Appl Physiol Respir Environ Exerc Physiol. 1982;52(3):677–682.
- Witzmann FA, Kim DH, Fitts RH. Hindlimb immobilization: length-tension and contractile properties of skeletal muscle. J Appl Physiol Respir Environ Exerc Physiol. 1982;53(2):335–345.
- Bigland-Ritchie B. EMG/force relations and fatigue of human voluntary contractions. Exer Sport Sci Rev. 1981;9:75–117.
- Moritani T, Muro M, Nagata A. Intramuscular and surface electromyogram changes during muscle fatigue. J Appl Physiol (1985). 1986;60(4):1179–1185.
- Martin TP, Edgerton VR, Grindeland RE. Influence of spaceflight on rat skeletal muscle. J Appl Physiol (1985). 1988;65(5):2318–2325.
- Riley DA, Ilyina-Kakueva EI, Ellis S, Bain JL, Slocum GR, Sedlak FR. Skeletal muscle fiber, nerve, and blood vessel breakdown in space-flown rats. FASEB J. 1990;4(1):84–91.
- Herbison GJ, Jaweed MM, Ditunno JF. Muscle fiber atrophy after cast immobilization in the rat. Arch Phys Med Rehabil. 1978;59(7):301–305.
- Maier A, Crockett Jl, Simpson DR, Saubert IV CW, Edgerton VR. Properties of immobilized guinea pig hindlimb muscles. Am J Physiol. 1976;231(5 Pt. 1):1520–1526.
- Kim DH, Witzmann FA, Fitts RH. Effect of disuse on sarcoplasmic reticulum in fast and slow skeletal muscle. Am J Physiol. 1982;243(3):C156–C160.
- Bigland-Ritchie B, Johansson R, Lippold OC, Smith S, Woods JJ. Changes in motoneurone firing rates during sustained maximal voluntary contractions. J Physiol. 1983;340:335–346.
- Edwards RH, Hill DK, McDonnell M. Myothermal and intramuscular pressure measurements during isometric contractions of the human quadriceps muscle. J Physiol. 1972;224(2):58P–59P.
- Shul’zhenko EB, Will-Williams IF. [Possibility of carrying out prolonged water immersion by the method of “dry” submersion]. Kosm Biol Aviakosm Med. 1976;10(2):82–84.
- Krnjevic K, Miledi R. Failure of neuromuscular propagation in rats. J Physiol. 1958;140(3):440–461.
- Lännergren J, Westerblad H. Action potential fatigue in single skeletal muscle fibres of Xenopus. Acta Physiol Scand. 1982;129(3):311–318.
- Koryak Y. Methods of investigation of neuromyscular system of athlete [internet]. Moscow: IMBP; 1992.
- Koryak Y. Contractile properties of the human triceps surae muscle during simulated weightlessness. Eur J Appl Physiol. 1995;70(4):344–350.
- Koryak Y, Polyakov VV, Potsepaev AI, Martyanov VA. The research of dynamic capacity for work of peripheral neuromuscular system of athlete. In: Korobkov AV, editor. Physiological Grounds of Movement Control. Moscow: Academic Press. 1975. P. 73–74.
- Badalyan LO, Skvortsov IA. Clinical electromiography. Moscow: Medicine; 1986.
- Sica RE, McComas AJ. An electrophysiological investigation of limb-girdle and facioscapulohumeral dystrophy. J Neurol Neurosurg Psychiat. 1971;34(4):469–474.
- Skvortsov IA. Electroneuromyography. Technigue. Dynamics of recordings in ontogenesis. In: Badalyan LO, Skvortsov IA, editors. Clinical and Electroneuromuscular Study of Neuromuscular Diseases and Syndromes. Moscow; 1981. p. 37–71.
- Merton PA. Voluntary strength and fatigue. J Physiol. 1954;123(3):553–564.
- Koryak Y. A comparison of the contractile properties in human leg muscles. Physiol J. 1984;40:30–38.
- White MJ, Davies CT. The effects of immobilization, after lower leg fracture, on the contractile properties of human triceps surae. Clin Sci (Lond). 1984;66(3):277–282.
- St. Pierre D. Gardiner PF. Effect of “disuse” on mammalian fast-twitch muscle: joint fixation compared with neurally applied to retrodoxin. Exp Neurol. 1985;90:635–651.
- Bezanilla F, Caputo C, Gonzalez-Serratos H, Venosa RA. Sodium dependence of the inward spread of activation in isolated twitch muscle fibres of the frog. J Physiol. 1972;223(2):507–523.
- Stalberg E. Propagation velocity in human muscle fibers in situ. Acta Physiol Scand Suppl. 1966;287:1–112.
- Desmedt JE. Methodes d’etute de la fonction neuromusculaire chez l’homme: myoframme isometrique, electromyogramme d’excitation et topographie de l’innervation terminale. Acta Neurol Psychiatr Belg. 1958;58:977–1017.
- Desmedt JE, Emeryk B, Renoirte P, Hainaut K. Disorder of contraction processes in sex-linked (Duchenne) muscular dystrophy, with correlative electromyographic study of myopathic involvement in small hand muscles. Am J Med. 1968;45:853–872.
- Milner-Brown HS, Miller RG. Muscle membrane excitation and impulse propagation velocity are reduced during muscle fatigue. Muscle Nerve. 1986;9:367–374.
- Khristova LG, Gidikov AA, Aslanova IF, Kirenskaya AV, Kozlova VG, Kozlovskaya IB. Effect of immersion hypokinesia on some parameters of human muscle potentials. Kosm Biol Aviakosm Med. 1986;20(6):27–31.
- Ruzzier F, Gregorio F Di, Scuka M. On the changes of the time course of the end-plate current during repetitive stimulation. Pflug Arch. 1982;393(1):121–132.
- Bianchi CP, Narayan S. Muscle fatigue and the role of transverse tubules. Science. 1982;215(4530):295–296.
- Noskov VB, Kozyrevskaya GI, Morukov BV, Artamasova EM, Rustamyan LA. Body position during hypokinesia and fluid-electrolyte metabolism. Kosm Biol Aviakosm Med. 1985;19:31–34.
- Witzmann FA, Kim DH. Fitts RH. Effect of hindlimb immobilization on the fatigability of skeletal muscle. J Appl Physiol Respir Environ Exerc Physiol. 1983;54(5):1242–1248.
- Woods JJ, Furbush F, Bigland-Ritchie B. Evidence for a fatigue-induced reflex inhibition of motoneuron firing rates. J Neurophysiol. 1987;58(1):125–137.
- Sandow A. Excitation-contraction coupling in skeletal muscle. Pharmacol Rev. 1965;17(3):265–320.
Keywords
“dry” Water immersion; Human triceps surae; Isometric intermittent contraction; Surface action potential; Muscle fatigue
Cite this article
Koryak YA. An electrophysiological investigations of the human triceps surae muscle after a simulated microgravity environment. Clin Case Rep J. 2022;3(2):1–10.
Copyright
© 2022 Yuri A Koryak. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-4.0).








