Palatal Lesions of Reactive Arthritis Triggered by a New Coronavirus: A Case Report
* Ana Glavina;
Džaja K;
Družijanić A;
Radić M;
-
* Ana Glavina: Department of Oral Medicine and Periodontology, Dental Clinic Split, University of Split, Split, Croatia.
-
Džaja K: Department of Oral Medicine and Periodontology, Dental Clinic Split, University of Split, Split, Croatia.
-
Družijanić A: Department of Oral Medicine and Periodontology, Dental Clinic Split, University of Split, Split, Croatia.
-
Radić M: Division of Rheumatology and Clinical Immunology, Center of Excellence for Systemic Sclerosis in Croatia, University Hospital Split, Split, Croatia.
-
Feb 24, 2022 |
-
Volume: 3 |
-
Issue: 2 |
-
Views: 6911 |
-
Downloads: 2509 |
Abstract
Background: The available scientific data on the oral manifestations of the new coronavirus is contradictory. Some scientists report that the oral and nasal mucosa is particularly affected during SARS-CoV-2 infection. However, others report infrequent involvement of the oral mucosa. Unlike oral lesions, orofacial symptoms such as anosmia/hyposmia and/or ageusia/dysgeusia/hypogeusia are better documented. We want to describe oral lesions in a patient with confirmed SARS-CoV-2 infection and associated comorbidities (i.e., reactive arthritis).
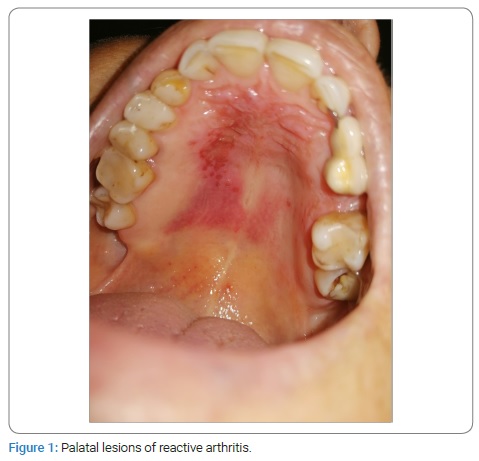
Case Presentation: We present oral symptoms and signs in a 57-year-old female patient with confirmed SARS-CoV-2 infection. The patient developed a milder clinical form of the disease in November 2020. Her medical history revealed that she had had reactive arthritis for the past nine years. Clinical oral examination showed bilateral erythema with multiple shallow erosions that were visible on the anterior part of the hard palate mucosa. There were no changes to the skin and other mucous membranes. For two weeks, she was prescribed local therapy with oral antiseptic and dexamethasone drops.
Conclusions: Erythema and erosions on the hard palate mucosa are oral manifestations of reactive arthritis triggered by immunosuppression during SARS-CoV-2 infection. The immune imbalance caused by COVID-19 requires a close long-term follow-up.
Abbreviations
SARS-CoV-2: Severe Acute Respiratory Syndrome Coronavirus-2; ACE2 receptors: Angiotensin-Converting Enzyme-2 Receptors; COVID-19: Coronavirus Disease 2019; ReA: Reactive Arthritis; Real-time RT-PCR: Real-time Reverse Transcriptase Polymerase Chain Reaction; RNA: Ribonucleic Acid; CBC: Complete Blood Count; ANA: Antinuclear Antibodies; ENA: Extractable Nuclear Proteins; HLA: Human Leukocyte Antigen; HSV: Herpes Simplex Virus; SLE: Systemic Lupus Erythematosus; OLP: Oral Lichen Planus; EM: Erythema Multiforme.
Introduction
The amount of available scientific data on the oral lesions of the new coronavirus Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) is increasing every day. It is mainly presented through case reports and case series. A few recent systematic reviews have been published to provide us with accurate information on the association between oral lesions and the new coronavirus. The available scientific data is contradictory. Some scientists report that during SARS-CoV-2 infection, the oral and nasal mucosa is particularly affected because of a cytokine storm [1]. The new coronavirus binds to Angiotensin-Converting Enzyme-2 (ACE2) receptors located on the epithelial cells of the oral mucosa and gingiva [2]. Others report infrequency involvement of the oral mucosa [3] and the absence of oral lesions in milder clinical forms of the disease [4]. Orofacial symptoms such as anosmia/hyposmia and/or ageusia/dysgeusia/hypogeusia are better documented than oral lesions. Therefore, they may raise the suspicion of early infection. It has been proven that anosmia and ageusia are caused by inflammation that occurred in COVID-19 [5]. The tongue is the most affected segment of the oral cavity due to the higher ACE2 receptors in its epithelial cells than the cheek mucosa or gingiva [2].
Our case report describes an extremely rare oral (palatal mucosa) manifestation of Reactive Arthritis (ReA) triggered by SARS-CoV-2. This is a case report of a non-classical form of ReA.
Case Presentation
We want to present oral symptoms and signs in a 57-year-old female patient with confirmed SARS-CoV-2 infection by Real-time Reverse Transcriptase-Polymerase Chain Reaction (real-time RT-PCR) amplification of the viral RNA. The patient developed a milder clinical form of the disease accompanied by fever (39ºC), shallow breathing, headache, myalgia, joint pain, hypogeusia, and hyposmia. She was prescribed antibiotic therapy with azithromycin tablets (500 mg, once daily for three days) due to the high fever (39ºC) that persisted for five days, after which her general condition improved. The patient noticed “changes in the oral mucosa” accompanied by a burning sensation and pain two weeks after the COVID-19 diagnosis. The symptoms prevented her from eating and drinking normally. Because of its anti-inflammatory, antioxidant, and antimicrobial effect, she rinsed her oral cavity with sage tea to no apparent effect.
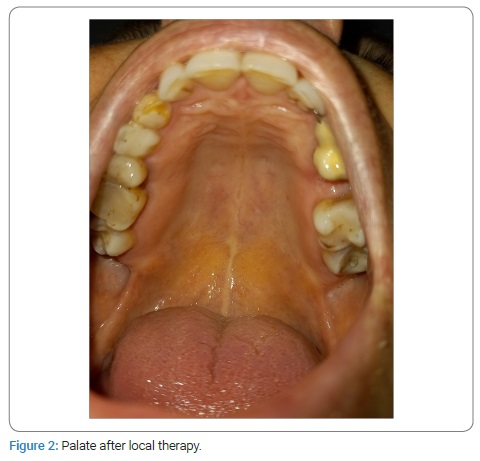
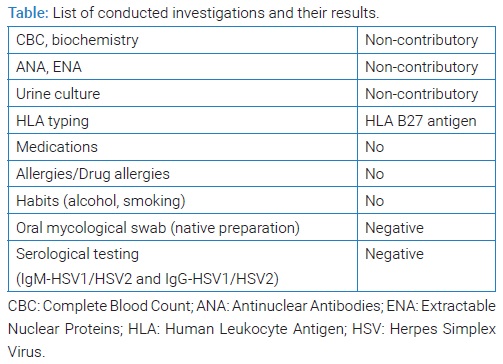
She stated that “similar lesions used to appear before in periods of immunosuppression.” These lesions would resolve spontaneously without medical intervention within two weeks. She also denied possible mechanical, chemical, and/or thermal injuries. Therefore, she consulted an oral medicine specialist at the Dental Clinic (teaching base of the School of Medicine, University of Split, study of Dental Medicine, Split, Croatia) in November 2020. Her medical history revealed that she has been suffering from ReA for the past nine years and from chronic gastritis. An epidemiological history revealed that she had not been vaccinated. She was not taking any medications. She had no allergies as well as drug allergies. She was a non-smoker. Her results of Complete Blood Count (CBC), biochemistry, Antinuclear Antibodies (ANA), Extractable Nuclear Proteins (ENA), and urine culture were non-contributory. Human Leukocyte Antigen (HLA) typing showed a positive finding of HLA B 27 antigen. She denied gastrointestinal and genitourinary problems. Clinical oral examination showed bilateral erythema with multiple, shallow erosions that were visible on the anterior part of the hard palate mucosa (Figure 1). An oral mycological swab was taken, and the native preparation was negative. She was also referred for serological testing for Herpes Simplex Virus (HSV) after three weeks due to possible asymptomatic primoinfection, although she denied recurrent HSV lesions. The results were also negative (Table). IgM-HSV1/HSV2 and IgG-HSV1/HSV2 antibodies usually appear at the same time, 10 days to 21 days after infection [6]. Dermato-venerological examination revealed no changes to the skin and other mucous membranes. A rheumatologist examination was also performed at the Division of Rheumatology and Clinical Immunology, University Hospital Split, Split, Croatia. She was prescribed local therapy with oral antiseptic (0.12% Chlorhexidine Digluconate and 0.05% Cetylpyridine Chloride, 2x daily) and dexamethasone drops (10 drops three times a day) for two weeks. Oral lesions remission occurred in ten days (Figure 2).
Discussion
ReA (previously called “Reiter’s syndrome”) occurs as a result of an abnormal immune response to one of the distant microbiological factors (i.e., gastrointestinal, genitourinary). Shigella flexneri, Shigella dysenteriae, Salmonella enteritidis, Salmonella typhimurium, Chlamydia trachomatis, Yersinia enterocolitica, Campylobacter jejuni, Clostridia difficile, Neisseria gonorrhoeae, and Ureaplasma urealyticum are well-known triggers. SARS-CoV-2 will certainly occur in the etiopathogenesis, and our case report makes a small scientific contribution. The association between ReA and SARS-CoV-2 needs to be established in further studies with more subjects. Case reports do not have much scientific value due to subjectivity and bias. The classical diagnostic triad for this syndrome consists of arthritis, non-gonococcal urethritis, and conjunctivitis, but most patients do not exhibit this typical clinical picture [7].
Oral lesions occur in 20% to 40% of the cases [8]. Papules and ulcerations on the buccal mucosa, gingiva, lips, and geographic tongue are the most commonly described oral lesions [7,8]. According to available data, as an oral manifestation of ReA, palate lesions have been described in two studies [9,10]. The differential diagnosis is broad and includes Systemic Lupus Erythematosus (SLE); oral allergic reactions (i.e., products containing cinnamon, volatile oils); oral bullous eruption caused by azithromycin; vitamin deficiency; Oral Lichen Planus (OLP); oral candidiasis; recurrent infections caused by HSV (herpes palati duri); mechanical, chemical and thermal injuries of the oral mucosa; erythroplakia; Erythema Multiforme (EM). We believe that bilateral erythema and shallow erosions on the hard palate mucosa in our patient are oral manifestations of ReA caused by the SARS-CoV-2 induced immunosuppression. Oral lesions occurred two weeks after SARS-CoV-2 infection, which is consistent with the time required for the development of ReA (several days to four weeks) [11]. Our patient also had a genetic predisposition, i.e., HLA B 27 antigen finding. Herpes palati duri mostly has unilateral localization. EM was excluded because the patient did not have the most common triggers (medications, HSV).
Conclusions
It is worth presenting this case of palatal lesions of Reactive Arthritis (ReA) because they rarely occur in everyday clinical practice. Therefore, they can easily be misdiagnosed and directly attributed to the Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) as the causative agent. Therefore, multidisciplinary collaboration, i. e., a collaboration of dermatovenerology, rheumatology, and oral medicine specialists, is important in making the final diagnosis of oral mucosal changes to provide patients with a timely therapeutic approach and eliminate unnecessary additional concern at the time of the Coronavirus Disease 2019 (COVID-19) pandemic.
Conflict of Interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Informed consent was obtained for this publication.
References
- Diamanti AP, Rosado MM, Pioli C, Sesti G, Laganà B. Cytokine release syndrome in COVID-19 patients, a new scenario for an old concern: The fragile balance between infections and autoimmunity. Int J Mol Sci. 2020;21(9):3330.
- Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12:8.
- Soares CD, Carvalho RA, Carvalho KA, Carvalho MG, Almeida OP. Letter to Editor: Oral lesions in a patient with Covid-19. Med Oral Patol Oral Cir Bucal. 2020;25(4):e563–e564.
- Vieira AR. Oral manifestations in coronavirus disease 2019 (COVID-19). Oral Dis. 2020;Suppl 3:770.
- Lechien JR, Chiesa-Estomba CM, Siati DRD, Horoi M, Bon SDL, Rodriguez A, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): A multicenter european study. Eur Arch Otorhinolaryngol Suppl. 2020;277(8):2251–2261.
- MCL Education. Discontinuation of Herpes Simplex Virus (HSV) IgM Testing [Internet]. Minnesota: MCL Education; 2019.
- Cheeti A, Chakraborty RK, Ramphul K. Reactive Arthritis. In: StatPearls [Internet]. Treasure Island (FL): Stat Pearls Publishing; 2021.
- Fotiou G, Laskaris G. [Reiter’s syndrome oral manifestations]. Hell Stomatol Chron. 1988;32(2):148–151.
- Michiels S, Kerre S. Palate Erosions in Reactive Arthritis. Arthritis Rheumatol. 2019;71(12):2089.
- Aitken-Saavedra J, Maturana-Ramirez A, Fernández Moraga J, Doro Dias V, Galdino-Santos L, Pineda Flores D. Reactive arthritis: images. Dermatol Online J. 2021;27(7):11.
- Toivanen A, Toivanen P. Reactive arthritis. Best Pract Res Clin Rheumatol. 2004;18(5):689–703.
Keywords
COVID-19; Reactive arthritis; Oral manifestations; SARS-CoV-2; Case report
Cite this article
Glavina A, Džaja K, Družijanić A, Radić M. Palatal lesions of reactive arthritis triggered by a new coronavirus: a case report. Clin Case Rep J. 2022;3(2):1–3.
Copyright
© 2022 Ana Glavina. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-4.0).




