Abstract
Colorectal cancer (CRC) is a common type of malignancy. However, metastatic CRC is rare and difficult to diagnose. We present a case of metastatic ovarian cancer mimicking primary rectal cancer. Our 76-year-old patient was diagnosed via immunohistochemical staining of an endoscopic biopsy sample and underwent surgery for low anterior resection and Hartmann’s procedure. With acceptable results, this was followed by chemotherapy based on cisplatin and paclitaxel regimens for ovarian carcinoma with colorectal metastasis.
Abbreviations
CRC: Colorectal Cancer; CT: Computed Tomography; CA125: Carbohydrate Antigens 125; CA19-9: Carbohydrate Antigens 19-9; CEA: Carcinoembryonic Antigen; CK7: Cytokeratin-7; PAX8: Paired Box Protein 8; CK20: Cytokeratin 20; CDX2: Caudal-type Homeobox 2; MRI: Magnetic Resonance Imaging; PET: Positron Emission Tomography; MUC2: Mucin 2; MUC5AC: Mucin 5AC; AMACR: Alpha-Methylacyl-CoA Racemase.
Introduction
Globally, colorectal cancer (CRC) ranks third among all malignancies [1]. In contrast, the overall lifetime risk of ovarian cancer is only 1.3%. However, approximately 60% of women with ovarian cancer have metastatic disease at the time of diagnosis [2]. Colorectal metastasis from the ovaries is rare. This case report describes a patient with an initial diagnosis of primary rectal cancer diagnosed as metastatic ovarian cancer, mimicking primary rectal cancer.
Case Presentation
A 76-year-old woman diagnosed with rectal cancer was hospitalized for surgery in December 2020. The patient’s medical history indicated type 2 diabetes mellitus and hyperlipidemia for 12 years; no other systemic diseases, cancer-related history, or family cancer history was noted. The patient first presented to the outpatient department with a chief complaint of tenesmus and hypogastric pain. On physical examination, her vital signs were normal. She had a soft abdomen, and no palpable mass was found. Lower abdominal tenderness was noted; however, there was no rebound tenderness. Laboratory testing revealed a white blood cell count of 10.31 × 103/μL, with a neutrophil-to-lymphocyte ratio of 77.7/14.3; other examinations, including liver function and renal function tests, were normal. Abdominal and pelvic Computed Tomography (CT) (Figure 1) revealed enlarged lymph nodes in the para-aortic region, bilateral iliac chains, left paracolic gutter, and right inguinal region; wall thickening of the sigmoid colon and rectum; pericolic fat stranding; outpouching structures with enlarged lymph nodes and fluid accumulation in the adjacent mesocolon. Gynecologic organs, such as the uterus and ovaries, were essentially normal under the current imaging study.
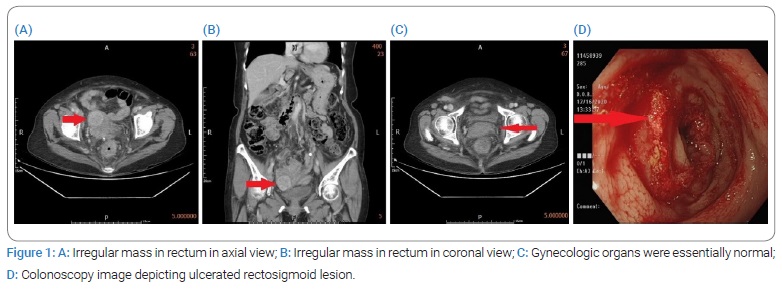
Colonoscopy (Figure 1) revealed a rectal tumor approximately 10 cm from the anal verge with nearly whole wall swelling of the sigmoid colon and rectum. An endoscopic biopsy was performed, and pathology revealed a moderately differentiated adenocarcinoma.
Laboratory testing revealed that the serum Carbohydrate Antigens 125 (CA125) level was elevated at 1061 U/mL, whereas the Carbohydrate Antigens 19-9 (CA19-9) and Carcinoembryonic Antigen (CEA) levels were normal. The patient underwent right inguinal lymphadenectomy, and the pathology results indicated a poorly differentiated metastatic adenocarcinoma.
Sudden onset of severe abdominal pain occurred during hospitalization, and repeated CT of the abdomen and pelvis revealed hollow-organ perforation with peritonitis. Diagnostic laparoscopy revealed rectal tumor perforation (Figure 2). Laparoscopy was converted to exploratory laparotomy with low anterior resection, and Hartmann’s procedure was performed. During surgery, the para-aortic lymph nodes and omentum were observed and excised. In addition, gynecologic organs, including ovaries, fallopian tubes, and uterus, were observed, where a right adnexal mass of about 1.5 cm in size was noted. All three excised lymph nodes revealed moderately to poorly differentiated metastatic adenocarcinoma as well as the omentum.
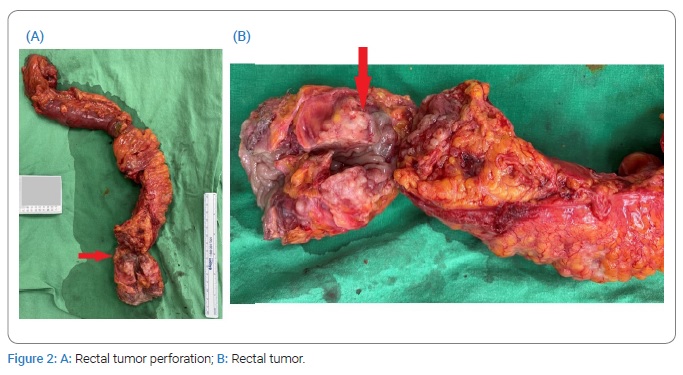
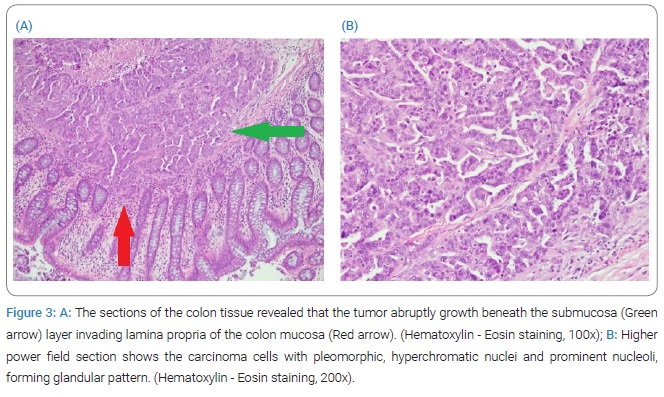
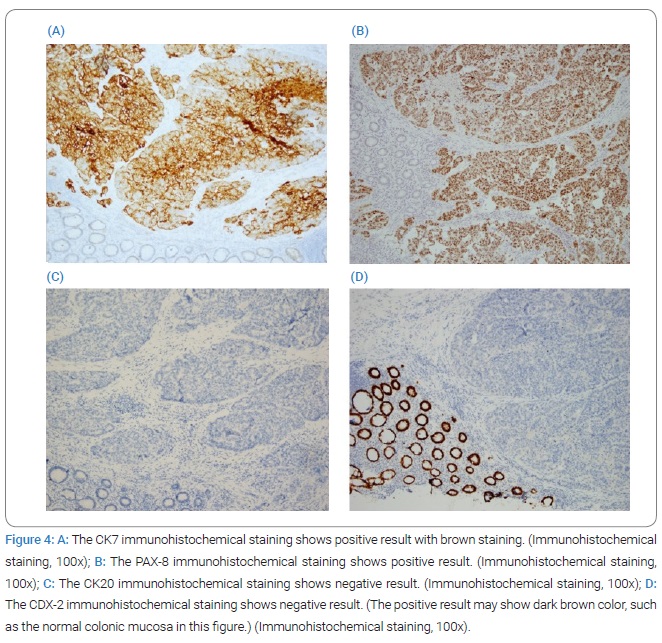
Pathological examination of the rectal tumor indicated moderately to poorly differentiated adenocarcinoma, invading from the mesentery to the mucosa of the colon, with regional lymphovascular invasion and metastasis in six out of seven dissected regional lymph nodes (Figure 3), with cutting end margins free of tumor invasion. Owing to elevated serum CA125 levels, immunohistochemical staining of the tumor was performed. Cytokeratin-7 (CK7) and Paired Box Protein 8 (PAX8) were positive, while Cytokeratin 20 (CK20) and Caudal-type Homeobox 2 (CDX2) were negative. The expression of this tumor indicated that it does not have a colonic origin (Figure 4).
Based on the surgical findings, immunohistochemical staining, and elevated serum CA125 levels, the tumor was suggestive of metastatic adenocarcinoma of the ovary.
The patient then received six cycles of adjuvant chemotherapy based on cisplatin and paclitaxel regimens for ovarian carcinoma with colorectal metastasis. The post-chemotherapy serum CA125 levels normalized to 16.89 U/mL. At the six-month follow-up post-chemotherapy, the serum CA125 level remained normal, and the CT scan showed no evidence of recurrence.
Discussion
Metastatic colorectal cancer occurs in only 1 percent of all colorectal cancer cases [3]. In patients with colorectal metastases, the absence of symptoms, such as bowel obstruction, hematochezia, vague abdominal discomfort, bowel perforation, and anemia, is common and may lead to delays in diagnosis. Endoscopically, metastatic colon tumors can protrude or ulcerate, increasing the difficulty of distinguishing between primary and metastatic colorectal adenocarcinomas.
In the large intestine, rectal cancer is the second most common type of cancer (28%) after proximal colon cancer (42%) [4]. In rectal cancer, sex plays a significant role [5]; different age groups also have different ratios of men to women incidence rates (1.10 for age < 49 years; 1.19 years, age 50 years–64 years; 1.27 50 years–79 years and 1.29 for age > 80 years) [4]. Statistically, women are diagnosed with rectal cancer at a median age of 65 and men at 63 [4].
To improve the diagnostic rate, adjunctive imaging techniques, such as sonography, CT, Magnetic Resonance Imaging (MRI), and Positron Emission Tomography (PET) scans, are helpful.
Rectal metastasis from ovarian cancer is rare, with only 5.97% of metastatic colorectal cancers being ovarian related in a Japanese autopsy study [6]. Accordingly, metastatic colorectal cancer can be easily mistaken for primary colorectal cancer, as demonstrated in our case. In addition, Colorectal cancer is typically treated with 5-fluorouracil and platinum agents [7], while ovarian cancer is generally treated with paclitaxel and platinum agents [8]; therefore, determining the origin of the cancer is crucial.
Tumor markers, including CEA, CA125, and CA19-9, may have additional roles in differentiating between primary colon cancer and ovarian metastasis, as well as in assessing treatment response. Nevertheless, serum CA125 levels may not be elevated in approximately 15% of patients with ovarian cancer [9]. Immunohistochemistry helps discriminate between tumor origins, such as those for CK7, CK20, CDX2, Mucin 2 (MUC2), Mucin 5AC (MUC5AC), and Alpha-Methylacyl-CoA Racemase (AMACR) and has been applied to differentiate between colonic-and ovarian-originating tumors [10,11]. CK7/CK-20 panels were used as benchmarks for differentiation. CK7 expression occurs in the lung, breast, ovary, and urothelium but rarely in the gastrointestinal tract. Conversely, CK-20 is commonly detected in most patients with colorectal cancer and Merkel cell tumors. It is also present in cases of pancreatic, gastric, and transitional cell cancers and cholangiocarcinomas but is virtually nonexistent in the lung, prostate, malignant mesothelioma, and non-mucinous ovarian cancers. Positive CK7 staining with negative CK-20 staining shows near-perfect specificity for ovarian tumors, and negative CK7 staining with positive CK-20 staining is 90% specific for colon tumors [9,12].
There is a wide variety of ovarian cancers with diverse manifestations. In most ovarian cancer cases, the disease is detected at an advanced stage; only 20% of cases were found in the ovaries at diagnosis [13]. Peritoneal seeding and/or direct invasion through colonic wall metastases are more common than the hematogenous or lymphatic spread in ovarian cancer. Approximately 70% of the patients who undergo laparotomy for staging have peritoneal metastases [14]. Consequently, the tumors first invade the colonic serosa and usually infiltrate inward [15,16], which is compatible with our case.
In conclusion, the distinction between primary and metastatic rectal carcinomas is crucial because the treatment and outcomes differ considerably, except for secondary colon metastases. Distinguishing between primary colon carcinoma and secondary metastasis is challenging. Colon metastasis should be suspected in patients presenting with newly developed gastrointestinal symptoms or new findings of colon cancer incidentally if they have a history of primary neoplasm.
Metastasectomy should be considered in cases of localized rectal metastasis. When gastrointestinal metastases from ovarian cancer are present, combined bowel resection with a 2 cm to 5 cm longitudinal margin of tumor burden and mesentery resection should be considered. This should include the intermediate and paracolic nodes and be conducted alongside chemotherapy or radiotherapy [17,18]. In secondary colorectal cancer, a patient’s overall survival depends on several factors, including the type of primary malignancy, the time at which metastasis is detected, the patient’s age, and follow-up [19].
Conclusion
In summary, we present a case of metastatic ovarian cancer mimicking primary rectal cancer. Proper evaluations, such as preoperative sonography, Computed Tomography (CT) and Magnetic Resonance Imaging (MRI) of the gastrointestinal tract and pelvis, and immunohistochemical staining, should be performed for ovarian and colorectal origin tumors to rule out any synchronous lesions to plan optimal treatment.
Acknowledgments
We would like to acknowledge Cheng-Ping Yu, for assistance with pathophysiology and microscopic images.
Author contributions: Shih-Hao Chou involved in literature review, study results interpretation, and manuscript editing. Ta-Wei Pu involved in study design and final editing. Jung-Cheng Kang and Cheng-Wen Hsiao and Chao-Yang Chen involved in study conception. Je-Ming Hu and Yen Hao and Hao-Tse Chiu contributed to manuscript draft. Kuan-Hsun Lin reviewed the literature.
Ethical approval: The project was approved by the Medical Ethics Committee of the Tri-Service General Hospital.
Consent: Written informed consent was obtained from the patient for the publication of this case report and accompanying clinical images.
Conflict of Interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Informed consent was obtained for this publication.
Keywords
Metastasis; Rectal cancer; Primary peritoneal; Serosal; CT; Immunohistochemical staining; Colorectal metastasis; Chemotherapy; Tumor marker; Colonoscopy
Cite this article
Chou SH, Pu TW, Hao Y, Chiu HT, Kang JC, Hsiao CW, et al. Metastatic ovarian cancer mimicking a primary rectal cancer: a case report. Clin Case Rep J. 2022;3(2):1–5.
Copyright
© 2022 Ta-Wei Pu. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-4.0).




