Cemiplimab: A Breakthrough Therapy for Advanced Cutaneous Squamous Cell Carcinoma
* Rubal Sharma;
Ralph Kamel;
Sandy El Bitar;
Sanyam Sharma;
Nishitha Thumallapaly;
Meekoo Dhar;
-
* Rubal Sharma: Department of Hematology and Oncology, Staten Island University Hospital, New York 10305, Unites States.
-
Ralph Kamel: Department of Internal Medicine, Staten Island University Hospital, New York 10305, United States.
-
Sandy El Bitar: Department of Hematology, Duke Hematology Clinic, North Carolina 27710, United States.
-
Sanyam Sharma: Department of Internal Medicine, Guru Nanak Dev Hospital, Amritsar 143001, India.
-
Nishitha Thumallapaly: Department of Hematology and Oncology, Conway Medical Center, South Carolina 29526, Unites States.
-
Meekoo Dhar: Department of Hematology and Oncology, Staten Island University Hospital, New York 10305, Unites States.
-
May 09, 2022 |
-
Volume: 3 |
-
Issue: 3 |
-
Views: 3628 |
-
Downloads: 2301 |
Abstract
Cutaneous Squamous Cell Carcinoma (cSCC) is the second most common type of skin cancer, characterized by the uncontrolled proliferation of keratinocytes. Treatment options depend on the stage of the disease, with systemic therapy reserved for locally advanced and metastatic disease. Cemiplimab, an anti-PD 1 monoclonal antibody, has proven efficacy against this highly immunogenic tumor, increasing evidence of its usefulness in unresectable, metastatic, and locally advanced diseases.
A 78-year-old female with no pertinent past medical history, a former 50-pack-per-day smoker, presented to the hospital for a large ulcerated right facial mass, weight loss, and anorexia. Physical exam showed a large 10 cm x 8 cm ulcerated, exophytic lesion with few areas of bleeding, involving the entire right side of the face with cervical erythema without fluctuance. A CT scan of the neck demonstrated a large heterogeneous ulcerated facial mass measuring approximately 9 cm x 10 cm x 3 cm, extending superiorly to the superior margin of the right orbit, inferiorly to the level of the maxilla, medially to the right nasal wall, and posteriorly to right maxillary sinus anterior wall with involvement of the infraorbital foramen with mildly prominent lymph nodes. A biopsy of the mass confirmed invasive, ulcerated, and poorly differentiated squamous cell carcinoma. A CT scan of the chest, abdomen, and pelvis and a whole-body nuclear bone scan was negative for distant metastatic disease. After a multidisciplinary meeting, the patient was deemed a poor candidate for surgical resection given the high morbidity or chemoradiation given the disease’s extent. A decision was made to administer Cemiplimab with the goal of tumor size reduction and eventual surgery. The patient received Cemiplimab 350 mg IV every three weeks, which was tolerated well with no reported side effects. The patient received 13 doses of Cemiplimab with a resolution of the mass and is pending a PET scan to assess for residual disease.
The above case illustrates the possibility of using Cemiplimab as a sole therapeutic option without the need for complementary reconstructive surgery or radiation therapy.
Introduction
Cutaneous squamous cell carcinoma (cSCC) is a malignant neoplasm of the skin, characterized by aberrant proliferation of keratinocytes with a high mutation burden [1]. It is the second most common non-melanoma neoplasm after basal cell carcinoma with increasing incidence and estimated mortality rates comparable to renal, oropharyngeal carcinomas, and melanomas in the southern and central United States [2]. Risk factors for the development of cSCC include ultraviolet exposure, old age, and immunosuppression [3]. Complete surgical resection is the recommended treatment for localized disease, with radiotherapy being performed for a non-resectable tumor or in patients who are poor surgical candidates or who decline surgery. Systemic therapy is reserved for locally advanced and metastatic (local and distant) cSCC, which cannot be treated with curative intent with either surgery and/or radiotherapy. Currently, given the lack of prospective randomized phase 3 trials comparing the efficacy and safety of chemotherapeutics, epidermal growth factor receptor (EGFR) inhibitors, and anti-programmed death 1 (anti-PD-1) antibodies, no systemic therapy has been approved for the treatment of locally advanced and/or metastatic disease [4]. Cemiplimab is a highly potent, high-affinity monoclonal antibody directed against (PD-1), which has shown efficacy in highly immunogenic advanced cSCC [5]. It showed an overall response rate of 50% in phase I/II trials, with a durable response exceeding six months in 57% of the responders [6]. There is increasing evidence to support its use as a sole agent for curative intent, as cases of complete remission after isolated treatment with Cemiplimab have been described in the literature [5,7]. We present a case of locally advanced cSCC of the face, with an excellent near-complete sustained response to Cemiplimab therapy without concurrent or subsequent treatment.
Case Presentation
An 80-year-old frail female, with no pertinent past medical history, former 50 pack-per-year smokers, presented to the hospital for a large ulcerated right facial mass (Figure 1), weight loss, and anorexia. History started one year before her presentation when she noticed a small lesion above the right nasolabial fold, rapidly growing over the last four months. Physical exam showed a large ulcerated, exophytic lesion, measuring approximately 8 cm x 8 cm (by measuring tape), with few areas of bleeding, involving the entire right side of the face, associated with cervical erythema without fluctuance or induration. A nasal speculum examination was performed and was limited because of the extrinsic compression of the nasal cavity but failed to reveal invasion of the mass into the nasal cavity.
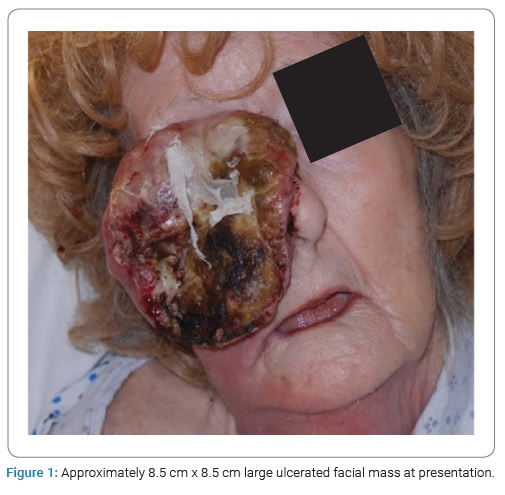
Admission laboratory studies were significant for iron deficiency anemia (Hemoglobin 7.8 g/dL [12 g/dL – 16 g/dL]) treated with a 3-day course of IV iron sucrose.
A CT scan of the head and neck demonstrated a large heterogeneous ulcerated facial mass measuring approximately 9 cm x 10 cm x 3 cm, extending superiorly to the superior margin of the right orbit, inferiorly to the level of the maxilla, medially to the right nasal wall, and posteriorly to right maxillary sinus anterior wall with involvement of the infraorbital foramen with mildly prominent lymph nodes (Figure 2).
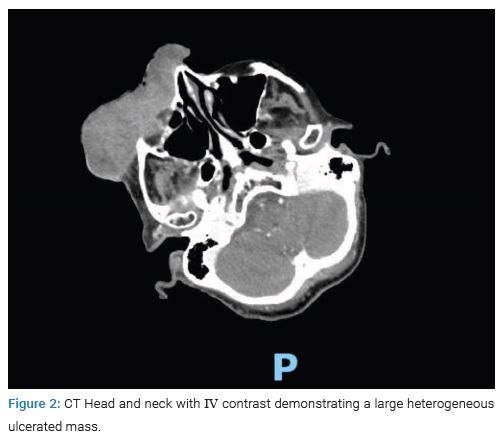
A biopsy of the mass was performed, and pathology reported invasive, ulcerated, and poorly differentiated squamous cell carcinoma. Immunohistochemical staining was focally positive for CK7, CD10, and CK5/6/P63 and was negative for S100, Melan A, MART-1, CK20, synaptophysin, chromogranin, CD56, ER, PR, GCDFP-15, and PAX-8.
Further workup with a CT scan of the chest, abdomen, and pelvis and a whole-body nuclear bone scan was negative for distant metastatic disease.
After a multidisciplinary meeting, the patient was deemed a poor candidate for surgical resection followed by reconstructive surgery given the high morbidity or chemoradiation given the disease’s extent. The decision was made to administer immunotherapy with Cemiplimab with the goal of tumor size reduction and eventual surgery. The patient was started on Cemiplimab 350 mg IV every three weeks and tolerated well with no reported side effects. After her fourth cycle, her tumor was significantly smaller in size (Figure 3).

At this publication, the patient had received 23 doses of Cemiplimab with near resolution of the mass. A plan to repeat a PET scan to assess residual disease is currently on hold as the patient was lost to follow-up.
Discussion
Most patients diagnosed with Cutaneous Squamous Cell Carcinoma have a limited-stage disease that is easily resectable at the time of diagnosis. Consequently, the condition is characterized by an elevated 5-year survival rate approaching 90%. However, despite the encouraging statistics, a clinically significant locally advanced disease is present in a significant subset of patients [8].
In our case, the patient presented with locally advanced squamous cell carcinoma with extensive infiltration of facial structures, making facial reconstructive surgery a poor therapeutic option because of the anticipated high risk of recurrence and the associated high peri-operative morbidity. Similar cases of cutaneous squamous cell carcinoma exist and are not amenable to standard curative treatments used for limited-stage diseases such as surgical resection, cryotherapy, and radiation therapy. Immunotherapy has been studied for this category of patients. One of the first categories of agents studied was EGFR inhibitors. Hillen et al. related the results of a retrospective study performed in 24 centers in Germany and Austria that included data from 190 patients with advanced cutaneous squamous cell carcinoma. The study showed that only two patients achieved complete response; however, a much higher proportion had a partial response and disease stabilization (27% and 43%, respectively) [9]. The advance of immune checkpoint inhibitors and their proven efficacy in treating other cancer types have led to their study as potential therapeutic options for patients with advanced cutaneous squamous cell carcinoma. Cemiplimab is a monoclonal antibody that binds to Programmed Death 1 (PD-1), preventing interaction with Programmed-Death Ligand 1 (PDL-1). It was approved in 2018 for the treatment of cSCC in patients who are not candidates for curative surgery or radiation [10]. The most popular ongoing study is the EMPOWER-CSCC1 phase 2 trial. It has been observed that Cemiplimab treatment was associated with a clinically objective response rate across two groups of patients: patients with locally advanced cSCC and patients with metastatic cSCC. It has been observed that responses appear durable and associated with a relatively good safety profile. The primary analysis shows that out of 78 patients, ten patients had a complete response after treatment with Cemiplimab [11]. Hermel et al. performed a retrospective analysis of 8 patients treated with at least one cycle of Pembrolizumab. Out of 8 patients that met inclusion criteria, 4 achieved a partial response, and 2 had stable disease [12]. It is worth noting that Cemiplimab is clinically useful for patients with locally advanced cutaneous squamous cell carcinoma in which curative surgery or radiation therapy are either impossible or, if performed, would lead to devastating cosmetic consequences. The literature has increasingly described cases of complete remission following Cemiplimab treatment. The most supportive study is a pilot phase II trial presented by Ferrarotto et al. that concluded that neoadjuvant immunotherapy in locoregionally advanced resectable cSCC is associated with a high pathologic response rate [13].
Conclusion
Based on this case report, we conclude that if a complete response to Cemiplimab is reported in the context of locally advanced cutaneous squamous cell carcinoma, and considering its relatively safe profile. It can potentially be considered as a sole therapeutic option without the need for complementary reconstructive surgery or radiation therapy if further data from randomized controlled trials support this conclusion.
Conflict of Interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Informed consent was obtained for this publication.
References
- Parekh V, Seykora JT. Cutaneous squamous cell carcinoma. Clin Lab Med. 2017;37(3):503–525.
- Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: Incidence, risk factors, diagnosis, and staging. J Am Acad Dermatol. 2018;78(2):237–247.
- Vaidya P, Mehta A, Ragab O, Lin S, In GK. Concurrent radiation therapy with programmed cell death protein 1 inhibition leads to a complete response in advanced cutaneous squamous cell carcinoma. JAAD Case Rep. 2019;5(9):763–766.
- Gellrich FF, Hüning S, Beissert S, Eigentler T, Stockfleth E, Gutzmer R, et al. Medical treatment of advanced cutaneous squamous-cell carcinoma. J Eur Acad Dermatol Venereol. 2019;33 Suppl 8:38–43.
- Migden MR, Rischin D, Schmults CD, Guminski A, Hauschild A, Lewis KD, et al. PD-1 Blockade with Cemiplimab in Advanced Cutaneous Squamous-Cell Carcinoma. N Engl J Med. 2018;379:341–351.
- Patel R, Chang ALS. Immune Checkpoint Inhibitors for Treating Advanced Cutaneous Squamous Cell Carcinoma. Am J Clin Dermatol. 2019;20(4):477–482.
- Migden MR, Khushalani NI, Chang ALS, Lewis KD, Schmults CD, Hernandez-Aya L, et al. Cemiplimab in locally advanced cutaneous squamous cell carcinoma: results from an open-label, phase 2, single-arm trial. Lancet Oncol. 2020;21(2):294–305.
- Soura E, Gagari E, Stratigos A. Advanced cutaneous squamous cell carcinoma. Current Opinion in Oncology. 2019;31(5):461–468.
- Hillen U, Leiter U, Haase S, Kaufmann R, Becker J, Gutzmer R, et al. Advanced cutaneous squamous cell carcinoma: A retrospective analysis of patient profiles and treatment patterns—Results of a non-interventional study of the DeCOG. Eur J Cancer. 2018;96:34–43.
- Markham A, Duggan S. Cemiplimab: first global approval. Drugs. 2018;78(17):1841–1846.
- Lee A, Duggan S, Deeks ED. Cemiplimab: A Review in Advanced Cutaneous Squamous Cell Carcinoma. Drugs. 2020;80(8):813–819.
- Hermel DJ, Ragab OM, Higgins S, Wysong A, In GK. PD-1 inhibition in cutaneous squamous cell carcinoma (cSCC). Journal of Clinical Oncology. 2018;36(15_suppl):e15100–e15100.
- Ferrarotto R, Amit M, Nagarajan P, Rubin ML, Yuan Y, Bell D, et al. Pilot Phase II Trial of Neoadjuvant Immunotherapy in Locoregionally Advanced, Resectable Cutaneous Squamous Cell Carcinoma of the Head and Neck. Clin Cancer Res. 2021;27(16):4557–4565.
Keywords
Cutaneous squamous cell carcinoma; Cemiplimab; Complete surgical resection
Cite this article
Sharma R, Kamel R, El Bitar S, Sharma S, Thumallapaly N, Dhar M. Cemiplimab: a breakthrough therapy for advanced cutaneous squamous cell carcinoma. Clin Case Rep J. 2022;3(3):1–4.
Copyright
© 2022 Rubal Sharma. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-4.0).



