Abstract
Vaccines against Sars-CoV-2 (Severe acute respiratory syndrome coronavirus 2) have demonstrated a crucial role in overcoming the Covid-19 (coronavirus disease 2019) pandemic, and a worldwide vaccination campaign has started at the end of December 2020. However, those subjects who exhibit contraindications to vaccines are at risk of remaining excluded from immunization. A severe allergy to a vaccine or a vaccine component represents such a condition. Nevertheless, an accurate allergological evaluation and approach could allow a safe and effective vaccine administration. Shortly after introducing the anti-Sars-CoV-2 vaccine (SCoVV), the first cases of anaphylaxis were described. The main allergen was identified in PEG-2000 (polyethylene glycol), which both mRNA vaccines contain, although to date, other mechanisms have been hypothesized. A great effort was required to manage those subjects considered at risk of allergic reactions to vaccines. Allergy guidelines, position papers, and case series and reports have been published to help clinicians during the vaccination campaign even with more complicated patients. Here we describe the case of a man suffering from a documented severe allergy to PEG and polysorbate, who was willing to undergo Sars-CoV-2 vaccination despite the risk identified. The choice of a desensitization protocol for SCoVV vaccine and premedication, including the anti-IgE monoclonal antibody omalizumab, permitted to safe immunization of the patient against Covid-19.
Introduction
Vaccines are considered one of the major public health achievements and they have contributed to saving millions of lives worldwide, long before and during the Sars-CoV-2 pandemic [1,2]. Furthermore, the massive adhesion to the vaccination campaign against Covid-19 has significantly decreased Covid-19-related morbidity and mortality in the population [1], highlighting the importance of the rapid availability of anti-Sars-CoV-2 vaccines SCoVV.
The opportunity of being immunized is often denied to those patients exhibiting contraindications to vaccines, such as a severe allergy to vaccine components. Nevertheless, allergists should always evaluate the proper vaccination approach to ensure safe and effective protection from diseases [3].
When the first reports of anaphylaxis to SCoVV occurred [4], a great apprehension regarding vaccine-associated allergic reactions arose worldwide. A major role was attributed to their excipients: PEG-2000 contained in both mRNA vaccines (Comirnaty®, Pfizer and BioNTech and Spikevax, Moderna) and polysorbate 80 contained in both vector vaccines (Vaxzevria®, AstraZeneca and Ad26.COV2-S, Janssen) [5]. Both PEG-2000 and polysorbate-80 belong to the PEG-excipient family but have rarely been described to cause allergies, which are usually characterized by severe reactions [6].
Here we describe the case of a man suffering from a documented severe allergy to PEG and polysorbate and who was willing to undergo Sars-CoV-2 vaccination despite the identified risk.
Primary importance was given to safety when elaborating the immunization procedure. A relapse of anaphylaxis, the missing protection against new spreading variants of SARS-CoV-2, and a consequent demotivation were some of the main consequences of a therapeutic failure.
After evaluating possible risks and benefits, we proposed a desensitization procedure, including a premedication with omalizumab. The patient received and well-tolerated both doses of SCoVV under allergological surveillance.
Case Presentation
We introduce the case of MT, a 71-year-old male who presented with a history of three episodes of adverse drug reaction (ADR) to different pharmacological compounds and required immunization against Sars-CoV-2.
In 2011 the patient experienced anaphylactic shock (urticaria, dysphonia, hypotension) after administering an intra-articular injection of methylprednisolone (Depomedrol®, contains PEG 3350). A few months later, a single pill of ciprofloxacin (Ciproxin®, which contains PEG 3350) provoked urticaria and hypotension within a few minutes. Subsequently, during a radiological test with an oral contrast medium, amidotrizoate (Gastrografin®, which contains polysorbate 80), he experienced an adverse reaction characterized by dyspnea.
At that time, he underwent allergological evaluation with skin testing, which resulted positive for Depomedrol®, as well as a drug provocation test with a PEG-based laxative, which was interrupted for urticaria after the intake of 1/100 of the total dose, leading to a diagnosis of PEG/polysorbate allergy.
Of note, he also complained of itchy, erythematous, and swollen soft tissues of the mouth after using PEG-containing toothpaste and mouthwash. However, after avoiding these excipients, he never relapsed any symptoms. No other comorbidities emerged. Sex, age, Italian, and international epidemiological scenario suggested undergoing vaccination against SARS-CoV-2.
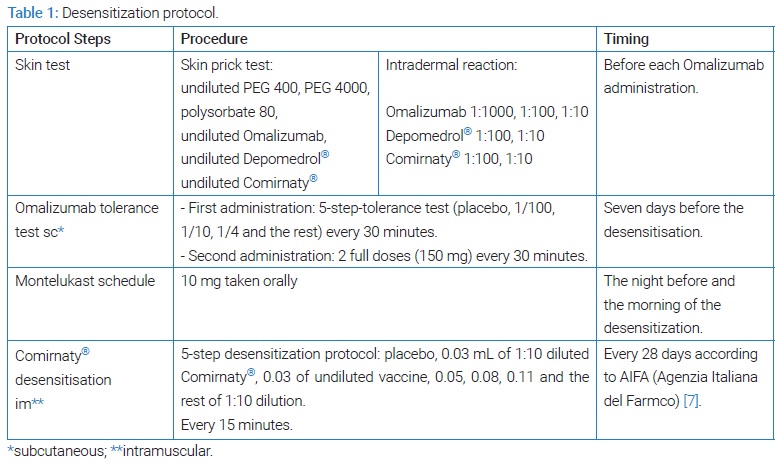
The patient expressed a strong desire to be immunized against Sars-CoV-2, and a risk-benefit analysis led to the proposal of a desensitization procedure with a vaccine product under allergological surveillance. At the time of the procedure, only mRNA vaccines containing PEG 2000 were available for mass immunization in Italy [7], and our Unit had Comirnaty®. To date, desensitization represents the safest mode to administer a drug that was confirmed to cause allergic reactions [8]. The need for rapid achievement of immunization and the estimated high risk of anaphylaxis led us to proceed with a robust premedication. Besides antihistamines and anti-leukotriene, omalizumab was of choice. However, it is content of polysorbate 20 [9], potentially cross-reactive with polysorbate 80 and PEGs [6], requires additional steps prior to immunization (Table 1).
The patient was given details regarding the whole procedure and the associated risks, and we obtained written informed consent for each diagnostic test, including desensitization. Skin testing was performed with PEG/polysorbate excipients, omalizumab (Xolair®, Novartis), and the Comirnaty® vaccine. Dilutions of drugs followed international guidelines and available literature [10,11]. Skin testing with the drugs mentioned above and excipients resulted in negative.
Omalizumab 300 mg was administered one week before each desensitization procedure, and the patient remained in observation for two hours following administration. The first time omalizumab was administered, a multi-step modality was chosen (Table 1), and no adverse events were registered. Considering the persistent negative skin tests to the monoclonal antibody one month prior to the second administration, omalizumab was successfully injected in only two steps, 150 mg each, with a 30-minute interval between doses. In addition, one tablet of montelukast 10 mg was taken the night before and the morning of the vaccination as this drug does not contain at-risk excipients [12].
A desensitization protocol was designed according to available literature and recent publications [10] (Table 1). After vaccine desensitization, the observation period was 3 hours.
The desensitization procedure was successful for both vaccine doses, allowing the patient to reach full immunization against SARS-CoV-2. The patients were tested for SARS-CoV-2 receptor-binding domain (RBD) of spike protein S1 subunit-reactive Immunoglobulin G (S1-RBD, Termofisher®) four weeks after the first and the second dose, reaching 14 BAU WHO/mL and 4920 BAU/mL respectively.
Discussion
Regarding vaccines, the Allergist’s prominent role consists of ensuring a safe vaccination in those subjects with known risk factors for allergic reactions (Table 2) [3]. Considering the importance of vaccines and safe immunization, a more personalized workup could be required for selected cases.

Our patient received a diagnosis of PEG allergy in 2012, based upon a history of anaphylaxis after exposure to PEG-containing drugs and positive diagnostic skin and provocation tests with PEGs. All these data were strongly suggestive of the existence of circulating IgE to PEGs, opening the possibility of a potential adverse reaction against the PEG-2000 contained in mRNA vaccines.
The negative result of the skin tests we encountered could not predict a potential tolerance towards the vaccine. It is well known that IgE antibody levels and consequently the probability of skin test positivity decrease overtime after an allergic drug reaction, but sensitization may persist for years [13].
Moreover, PEG is a non-protein allergen whose sensitization in allergic subjects is unpredictable over time and, therefore, compared to classic protein allergens [6]. The most documented data regarding PEGs is their risk of inducing severe reactions, primarily when contained in injectable drugs [6].
An immunization with SCoVV in such conditions was problematic, and according to international guidelines, we could have proceeded in exempting this patient from anti-Sars-CoV-2 vaccination [11]. However, his strong will to be protected against Covid-19, his potential health risks in case of infection, and the pandemic situation due to the emerging highly contagious Omicron [14] variant, led us to decide to attempt vaccination. Therefore, in our opinion, the risk/benefit analysis favored vaccination.
After consulting international literature, supporting data regarding potential safe immunization emerged.
Among thirteen subjects with documented PEG allergy belonging to a cohort described by Brockow et al., seven received SCoVV [15]. Most of them tolerated a complete course of Vaxzevria® (containing polysorbate-80); Comirnaty® was accidentally administered elsewhere to two subjects without any safe mode but nevertheless without registering any reaction [15]. None of them underwent desensitization [15].
These data are of great interest, although based on a small number of patients. Tolerance towards an injectable medication containing PEG could depend on the presence of very small amounts of PEG-2000 in mRNA SCoVV and on the lack of cross-reactivity between PEG and polysorbate-80 when viral-vector SCoVV was of choice [15]. Moreover, PEG allergic patients could tolerate PEGs with lower molecular weight than the culprit [6]; hence, IgE reacting against PEG-6000 may not recognize PEG-3350 or PEG-2000 or lower when tested [6].
Of note, a proven type 1 hypersensitivity to PEGs rarely emerges in case of suspected allergic reactions to SCoVV [5,16,17]. Even when tests turn positive, many authors have noted a good tolerance toward a second dose of SCoVV [17,18]. In a study by Worm et al., some patients with positive allergy tests to PEG and a positive history of vaccine (both anti-Covid-19 and not anti-Covid-19) hypersensitivity reactions tolerated SCoVV and even the same implicated in the previous adverse event [16]. These results have been partially attributed to a limited value of skin tests [16]; for example, in one study, the use of Refresh Tears® for a skin prick test resulted in irritants [17]. The value of skin tests for suspected PEG allergies is still debated [5]. The timing of positive skin testing is rarely addressed for vaccine skin tests; it is well known that they frequently turn positive, mainly in a delayed fashion, lacking in sensibility [3]. This phenomenon, similar to that of the Mantoux test, requires a precise interpretation of the results according to the patient’s history [3].
Other mechanisms different from PEG allergy probably play a significant role in eliciting allergic reactions to SCoVV. PEGs can activate mastocytes through pathways different from IgE cross-linking on FcER1 [15]. It has also been speculated that other parts of SCoVV cause reactions even at the first exposure to the vaccine [10,19]. Of note, most subjects who undergo a second dose of SCoVV after a suspected allergic reaction after the first dose do not relapse with an adverse reaction [5]. Subjects exhibiting positive BAT test for SCoVV tolerated a second dose [18].
Consequently, the proposal of a re-exposure [20] to SCoVV even in patients with PEG and polysorbate allergy has become plausible [21].
In our case, desensitization was the protocol of choice, as it is the safest mode to reintroduce culprit drugs to subjects with confirmed IgE mediated hypersensitivity reactions (HSR) [8]. In addition, the tolerance toward the drug is transient, and a re-administration without a desensitization procedure remains contraindicated [8]. International literature reports a high success rate with antibiotics, chemotherapeutics, biologicals, and other drugs [8]. In 2013 Kelso et al. published the protocol for vaccine desensitization [22]. The scheme refers to vaccines with a traditional injected volume of 0.5 mL [22]; the new ENDA/EAACI guidelines propose an adapted desensitization scheme for Comirnaty®, whose quantity is 0.3 mL per dose [10].
Although desensitization induces a temporary state of tolerance towards compounds responsible for HSR [23], mastocyte-mediators could accidentally be released, inducing adverse reactions.
The great experience with chemotherapeutics and biologics desensitization suggests using a premedication consisting of antihistamines and leukotriene blockers [24]. However, there is no consensus on premedication before all desensitizations, which should be evaluated case per case [8]. Antihistamines (H1 and H2 blockers) avoid histamine release from mastocytes, and leukotriene blockers act against end-products of the arachidonic acid cascade [25]. Of note, corticosteroids are not indicated in avoiding HSR [25]. Nevertheless, these drugs sometimes do not avoid anaphylaxis breakthroughs, mainly if ADA (specific anti-drug antibodies) belonging to the IgE isotype exists [8].
Moreover, corticosteroids are immunosuppressants, and when associated with vaccines, they could interfere with the physiological immunizing process. Huynh VA et al. performed effective desensitization to Comirnaty®, adopting a similar desensitization procedure but choosing a premedication with prednisolone and antihistamines [26]. Nevertheless, the capability of a single dose of prednisolone to hamper immunization after a vaccine could be not significant.
Hence, we encountered many possible risks of failing desensitization when considering our patients. As mentioned above, exposure to PEG in sensitized subjects usually leads to severe reactions, particularly to injectable drugs [6]. Our patient had experienced anaphylaxis to PEGs but avoiding the vaccination against SARS-CoV-2 exposed him to the risk of Covid-19 infection and its complications. The protocol for Comirnaty® desensitization is relatively new and not based on PEG allergy since no literature regarding PEG desensitization exists [10]. Patrawala M et al. performed successful desensitization to Pegvilase®; PEG was thought to be the culprit of anaphylaxis, but no confirmatory allergy tests were performed [27].
So a more robust premedication before SCoVV desensitization seemed advisable.
Hence, we chose to reach the highest level of safety by blocking Immunoglobulin E with omalizumab, which has demonstrated great efficacy in high-risk desensitization [28].
Patients experiencing anaphylaxis at the first attempt of desensitization to Hymenoptera venom tolerated a second course when premedicated with omalizumab [29]. The monoclonal antibody has a prominent role in food desensitization protocols, exhibiting a low failure rate [30]. In a recent review, Fernandez et al. listed the articles regarding the successful role of omalizumab in desensitization to drugs such as chemotherapeutics, insulin, and acetylsalicylic acid [23]. This approach is rare regarding drug allergies but could be life-saving [31]. The dose of omalizumab administered was usually 300 mg, 7 days–15 days before the drug desensitization [23].
Furthermore, allergic reactions to omalizumab are rare [32], although in some cases, its excipient polysorbate 20 could be considered responsible [33]. Our patient reported a suspected reaction against polysorbate 80 of Gastrografin® (diatrizoic acid), and polysorbate 80 could cross-react with polysorbate 20 [6]. Therefore, a complete allergological evaluation, including a tolerance test to omalizumab, was mandatory, although scientific experience is very limited.
Finally, regarding effectiveness, the patient exhibited an immunological response to the vaccine considering the significant increase in antibodies against S1-RBD after the second dose. However, the required titer to gain protection against Covid-19 is still lacking, although it is plausible that the presence of neutralizing antibodies represents a degree of protection [34].
Cite this article
Radice A, Fassio F, Meucci E, Bormioli S, Di Scala G, Macchia D. Omalizumab-adjuvanted desensitization to Comirnaty® in a patient with previous PEG anaphylaxis. Clin Case Rep J. 2022;3(4):1–6.


