Pancreatic Solid-Pseudopapillary Neoplasm in an Adolescent Girl Presenting as an Acute Abdomen
Gudumac Eva;
* Jana Bernic;
Livsit Irina;
Virgil Petrovich;
-
Gudumac Eva: “Natalia Gheorghiu” Department of Pediatric Surgery, Orthopedics and Anesthesiology, “Nicolae Testemitanu” State University of Medicine and Pharmacy of the Republic of Moldova, Chisinau, Moldova.
-
* Jana Bernic: “Natalia Gheorghiu” Department of Pediatric Surgery, Orthopedics and Anesthesiology, “Nicolae Testemitanu” State University of Medicine and Pharmacy of the Republic of Moldova, Chisinau, Moldova.
-
Livsit Irina: “Natalia Gheorghiu” Department of Pediatric Surgery, Orthopedics and Anesthesiology, “Nicolae Testemitanu” State University of Medicine and Pharmacy of the Republic of Moldova, Chisinau, Moldova; “Natalia Gheorghiu” National Scientific and Practical Center for Pediatric Surgery, Research Institute for Mother and Child Health Care, Chisinau, Moldova.
-
Virgil Petrovich: “Natalia Gheorghiu” Department of Pediatric Surgery, Orthopedics and Anesthesiology, “Nicolae Testemitanu” State University of Medicine and Pharmacy of the Republic of Moldova, Chisinau, Moldova; “Natalia Gheorghiu” National Scientific and Practical Center for Pediatric Surgery, Research Institute for Mother and Child Health Care, Chisinau, Moldova.
-
Jun 17, 2022 |
-
Volume: 3 |
-
Issue: 4 |
-
Views: 2334 |
-
Downloads: 2065 |
Abstract
A rare clinical case of the pediatric solid-pseudopapillary tumors of the pancreas is described. The diagnostic techniques and the choice of surgical procedures and adjuvant chemotherapy, using own experience, are presented. In addition, the literature data that reveal many novelties regarding the complications and the exodus of the disease is discussed.
Introduction
Recent studies show that the incidence of pancreatic cancer in children has increased alarmingly in recent decades, especially in economically developed countries [1]. This may be due to the influence of certain epigenetic and environmental risk factors [2,3]. However, how environmental factors interact with the cells of the growing organism, particularly those of the pancreas, and the etiopathogenic factors involved in developing pancreatic tumors are not yet fully known.
Solid-pseudopapillary neoplasm, Pseudopapillary Solid Tumor (PST), or infantile papillary epithelioma is an extremely rare cystic epithelial tumor with a low potential for malignancy [4,5]. According to data reported by Lee et al. [6], the frequency of tumor localization differs in children and adults: in children, the pancreatic head is affected (66.7%); In adults, the body or tail (80.9%). The tumor was first reported as a separate nosological form by L. Lichtenstein in 1933 and diagnostically confirmed in 1959 by VK Frantz [7,8].
Studies show that solid-pseudopapillary neoplasm can have a severe clinical evolution, especially by invading the surrounding tissues. Complications of the tumor may include intestinal obstruction at the duodenum or colonic intestine level, hemorrhagic disorders, manifested by hemorrhage resulting from the erosion of a blood vessel, a fact often encountered in our clinical practice in various abdominal tumors. Other complications include wound and abdominal infection, pancreatitis, pancreatic fistulae, and mortality [9]. In the case of liver metastases, the tumor can cause cholestatic jaundice due to obstruction of the intrahepatic bile ducts. If the tumor destroys enough islet cells in the pancreas, it can cause diabetes [10]. The tumor can be located at various levels of the pancreas [11].
Considering the rarity of the pancreas in children, the difficulties of diagnosis, and differential diagnosis, reporting each new clinical case is of undoubted practical interest.
Case Presentation
The 16-year-old female child from a rural area was admitted to “Natalia Gheorghiu” National Scientific and Practical Center for Pediatric Surgery, Research Institute of Mother and Child Care of Moldova, with the diagnosis of the acute surgical abdomen, having an onset of the disease six months before. From the antecedents, we mention that the child has been considered ill since June 2021, so two months ago, when the abdominal pains and bloating appeared, to which the lack of appetite, constipation, and insignificant weight loss was added. This semiology began two months ago, until the doctor’s consultation. The patient did not receive treatment. Then, two months after the disease onset, the doctor was consulted, and an abdominal tumor was found on clinical examination. Abdominal ultrasound (Figure 1) confirmed the presence of 2 abdominal tumors: a cystic lesion of the pancreas and an ovarian cyst; the latter was resolved by conservative treatment.
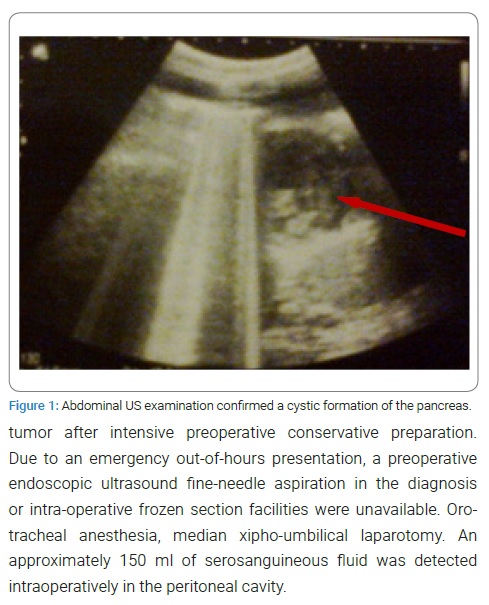
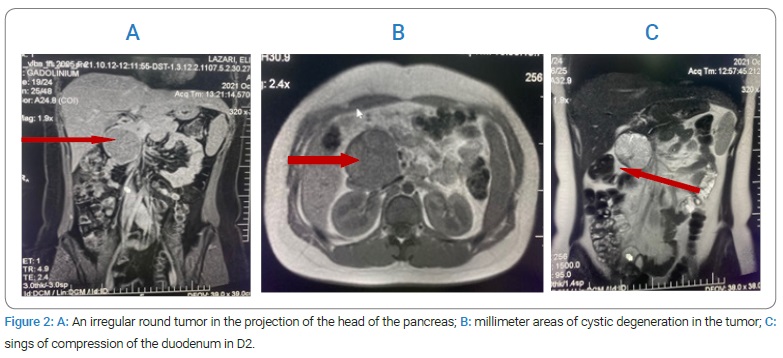
Six months after the disease onset, the patient was presented and admitted to the pediatric surgery department for permanent moderate abdominal pain, loss of appetite, and general weakness. In an abdominal Magnetic Resonance Imaging (MRI) (Figure 2) with contrast angiography in the region of the head of the pancreas, an irregular round tumor was found, well outlined with dimensions of 5.6 cm x 5.8 cm x 6.25 cm with millimeter areas of cystic degeneration, with signs of compression of the duodenum more accentuated in D2 of the vena cava, but without communication with the Wirsung canal.
Laboratory examinations are presented in (Table 1). The laboratory tests are essential for the clinician in terms of detecting the side effects of PChT, the consequences of which can be fatal for the patient.
Urinalysis, no pathological changes were revealed.
Surgery was performed after the diagnosis of retroperitoneal tumor after intensive preoperative conservative preparation. Due to an emergency out-of-hours presentation, a preoperative endoscopic ultrasound fine-needle aspiration in the diagnosis or intra-operative frozen section facilities were unavailable. Oro-tracheal anesthesia, median xipho-umbilical laparotomy. An approximately 150 ml of serosanguineous fluid was detected intraoperatively in the peritoneal cavity.
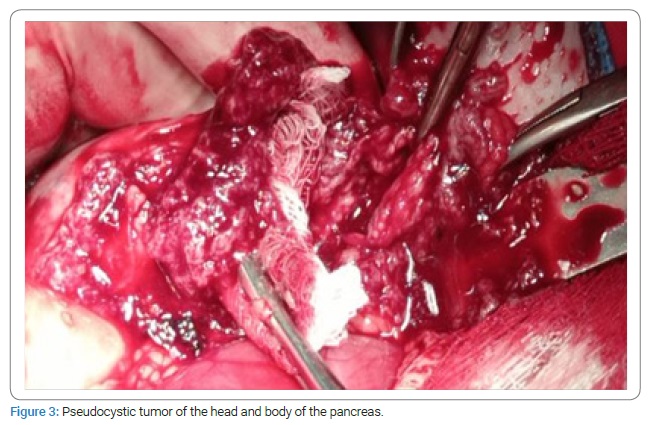
After carefully exploring the peritoneal cavity, a tumor formation was detected retroperitoneally. The omental bursa was opened, and a pseudocyst tumor (Figure 3) was found in both the head and body of the pancreas with the invasion of the duodenal wall at the level of D2–D3, as well as the surrounding retroperitoneal connective tissue, but also an advanced process of adhesion in the given area was observed.
Subtotal excision of the tumor was performed with technical difficulties. The postoperative evolution was complicated, being present as a grade III anemia and postoperative pancreatitis associated with pneumohydroperitoneum, which served as an indication for exploratory relaparotomy.
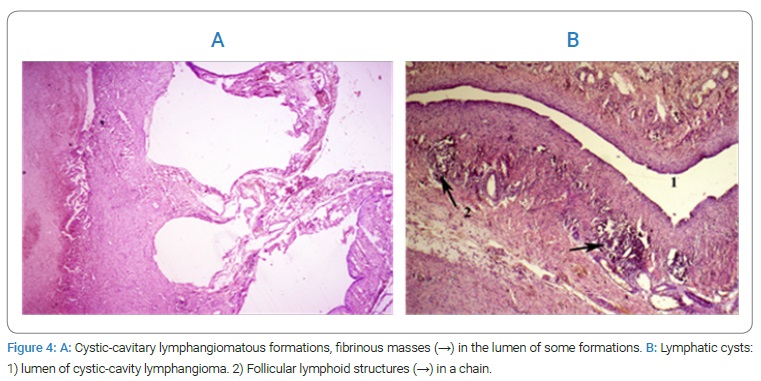
No gastrointestinal tract perforation was detected intraoperatively, and only a major adhesion process was present. Histopathological examination (Figure 4) of the tumor pieces revealed a solid-pseudopapillary tumor of the body of the pancreas.
The patient underwent intensive conservative treatment and, in a stable general condition, was transferred to the Oncological Institute for specific, adjuvant treatment, which included two blocks of Polychemotherapy (PChT): Cisplatin 90 mg-1 day, infusion 24 hours, Gemcitabine 1600 mg-1.8 day. The treatment was supported with toxic hepatitis, but the disease’s evolution was improved. Pharmaceutical treatments include sol. Glucose 5%, sol. Sodium chloride 0.9%, antioxidants, analgesics, nonsteroidal anti-inflammatory, antibacterial drugs, Mezym, Onaseron, Riboxin, Furosemide, Hepamethion, Silimarin, Ursodeoxycholic acid. The disease’s evolution improved, and the patient’s condition at discharge after one month was satisfactory. It is recommended to be readmitted to the Pediatric Oncology department to continue the PChT treatment.
Discussion
Pancreatoblastoma (PB) is an extremely rare pancreatic tumor that commonly occurs in infants and young children [12]. We present this case with reference to two elements of rarity: solid pseudopapillary tumors of the pancreas are tumors rarely found in surgical practice in children, and the given tumor form showed a very rare cause of acute surgical abdomen [3].
This is the second case of a solid pseudopapillary tumor of the pancreas in the practice of our pediatric surgery department.
The etiology of this tumor so far is not sufficiently deciphered. However, it is thought that this neoplasm may be derived from pluripotent pancreatic stem cells or from cells of female genital epithelial origin due to the increased prevalence in younger women [13].
A number of epidemiological studies have highlighted the direct link to other factors, such as the action of suppressor oncogenes (reactive genes) and proliferation inhibitors [14,15].
An oncogene is a mutated gene that has the potential to cause cancer. Before an oncogene becomes mutated, it is called a proto-oncogene. A significant difference between oncogenes and tumor suppressor genes is that oncogenes result from proto-oncogenes’ activation (turning on). However, tumor suppressor genes cause cancer when they are inactivated (turned off).
Cancer is driven by genetic and epigenetic alterations that allow cells to overproliferate and escape mechanisms that normally control their survival and migration. Many of these alterations map to signaling pathways that control cell growth and division, cell death, and cell motility and can be placed in the context of distortions of wider signaling networks that fuel cancer progression. Mutations that convert cellular proto-oncogenes to oncogenes can cause hyperactivation of these signaling pathways, whereas inactivation of tumor suppressors eliminates critical negative regulators of signaling [16]. Pharmacologic and antibody-based inhibitors targeting signaling proteins mutated in tumors or proteins downstream from these have significantly impacted cancer treatments [17–19]. In some cases, tumor formation results from the aberrant activity of various cell-cycle regulators that play essential roles in many signal transduction pathways. For example, abnormalities of the histone methyltransferase enhancer of zeste homolog 2 pathway, Hedgehog signaling pathway, mitogen-activated protein kinase pathway, and TGFβ pathway had the highest alteration rate in pancreatic cancers and are critical for the development of new therapeutic targeted approaches that can improve patient care [20–24].
Rare cases of localization in the peripancreatic tissue and in the middle of the colon are described (a fact present in our case). Often PST can develop asymptomatically, but at the same time, the child may have complaints of abdominal discomfort, feeling of a full stomach, nausea, pain in the epigastrium, in the upper abdomen, and very rarely is the clinic of the acute surgical abdomen or acute pancreatitis. In the case of total removal of the tumor, the 5-year survival is 95%; in its incomplete resection, it may recur [4,25]. Potentially predisposing factors for recurrence are the large volume of the tumor, its rupture, and the child’s young age. Metastases are present in 5%–10% of cases, especially in the liver and lymph nodes, but rarely in the peritoneum [26–28]. According to data reported by Papavramidis T and Papavramidis S [4], among patients with metastases, the most common location is the liver, portal vein, spleen, then other organs (duodenum, omentum, colon, lung, peritoneum). The prognosis of Solid Pseudopapillary Neoplasm (SPN) is remarkably good, even in the presence of metastasis, with an overall 5-year survival rate of about 97% [29]. Multidisciplinary treatment, including resection, may improve the prognosis of patients with SPN of the Pancreas with recurrence or metastasis [30].
In the present case, we suggest that a late diagnosis occurred when the tumor massively invaded the neighboring organs (duodenum, surrounding peripancreatic connective tissue). As our patient presented with an acute abdomen and out-of-hours emergency, endoscopic ultrasound fine-needle aspiration in the diagnosis or intra-operative frozen section facilities were unavailable; otherwise, a conventional duodenopancreatectomy would have been possible if a pre-operative diagnosis was available [31,32].
Studies suggest that surgical resection should be performed even in patients with widespread, advanced metastases, as these patients may have a long survival time [25,26].
In our case, the absence of liver metastases is characteristic, while in the cases analyzed by other researchers [3], almost half of the patients had liver metastases (16/35, 45.7%).
Conclusion
Pseudopapillary solid pancreatic tumor is a rare clinical feature in surgical practice in children. Statistics show that the solid pseudopapillary tumor of the pancreas most frequently affects females aged from 20 years to 30 years. The clinical picture is that of an abdominal disease, and the preoperative diagnosis is difficult and can be suggested by abdominal ultrasound, Computed Tomography (CT), or Magnetic Resonance Imaging (MRI) with angiography. The definite diagnosis was made by histopathological examination. The solid pseudopapillary tumor of the pancreas may begin with bleeding and digestive disorders. The treatment is complex - surgical and adjuvant (chemotherapy, radiotherapy, immunotherapy, and various combinations between them). We mention that whenever there are clinical signs, imaging data, or intraoperative findings that could raise the suspicion of an abdominal tumor, it is intervened by surgical treatment followed by histological examination, but if possible, initially extemporaneously (intraoperatively), followed by postoperative histological examination should be performed. The correct diagnosis of SPN in the pancreas depends on a comprehensive analysis of clinical, imaging, and histopathologic characteristics. Surgical resection should be performed and should be considered even in patients with advanced disease.
Authors’ Contributions
Gudumac Eva, Bernic Jana and Livsit Irina participated in the whole diagnostic and treatment process of the described patients. Gudumac Eva and Bernic Jana conceived the concept of the study. Bernic Jana and Livsit Irina collected the data. Virgil Petrovich participate in the histopathological examination and description. Gudumac Eva wrote the manuscris, and all authors contributed to revisions. All authors read and approved the final manuscript.
Ethics Committee Approval
Written informed consent was obtained from the patient legal guardian for publication of this case report and any accompanying images, both orally and in writing, in accordance with the declaration of Helsinki ethical principles for medical research involving human subjects.
Conflict of Interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Informed consent was obtained for this publication.
References
- Wong MCS, Jiang JY, Liang M, Fang Y, Yeung MS, Sung JJY. Global temporal patterns of pancreatic cancer and association with socioeconomic development. Sci Rep. 2017;7(1):3165.
- Capasso M, Franceschi M, Rodriguez-Castro KI, Pellegrino C, Ginevra C, Chiara M, et al. Epidemiology and risk factors of pancreatic cancer. Acta Biomed. 2018;89(Suppl 9):141–146.
- Liu T, Zhao T, Shi C, Chen L. Pancreatoblastoma in children: Clinical management and literature review. Transl Oncol. 2022;18:101359.
- Papavramidis T, Papavramidis S. Solid pseudopapillary tumors of the pancreas: review of 718 patients reported in English literature. J Am Coll Surg. 2005;200(6):965–972.
- Słowik-Moczydłowska Ż, Gogolewski M, Yaqoub S, Piotrowska A, Kamiński A. Solid pseudopapillary tumor of the pancreas (Frantz’s tumor): two case reports and a review of the literature. J Med Case Rep. 2015;9:268.
- Lee SE, Jang JY, Hwang DW, Park KW, Kim SW. Clinical features and outcome of solid pseudopapillary neoplasm: differences between adults and children. Arch Surg. 2008;143(12):1218–1221.
- Lichtenstein L. Papillary cystadenocarcinoma of pancreas: case report, with notes on classification of malignant cystic tumors of pancreas. The American Journal of Cancer. 1934;21(3):542–553.
- Frantz VK. Tumors of the pancreas. In: Atlas of tumor pathology, section 7, fascicles 27 and 28. Washington, DC: Armed Forces Institute of Pathology; 1959. p. 334.
- Guo T, Wang L, Xie P, Zhang Z, Yu Y. Diagnosis and Surgical Treatment and Pathological Findings of Solid Pseudopapillary Tumor of the Pancreas: A Single-Institution Experience. Cancer Manag Res. 2020;12:581–588.
- Kim MS, Park H, Lee S, Yoo SY, Cho SY, Lee SK, et al. Clinical characteristics, treatment outcomes, and occurrence of diabetes mellitus after pancreatic resection of solid pseudopapillary tumor in children and adolescents: A single institution experience with 51 cases. Pancreatology. 2021;21(3):509–514.
- Jena SS, Ray S, Das SAP, Mehta NN, Yadav A, Nundy S. Rare Pseudopapillary Neoplasm of the Pancreas: A 10-Year Experience. Surg Res Pract. 2021;2021:7377991.
- Gu WZ, Zou CC, Zhao ZY, Liang L, Tang HF. Childhood pancreatoblastoma: clinical features and immunohistochemistry analysis. Cancer Lett. 2008;264(1):119–126.
- Naar L, Spanomichou DA, Mastoraki A, Smyrniotis V, Arkadopoulos N. Solid Pseudopapillary Neoplasms of the Pancreas: A Surgical and Genetic Enigma. World J Surg. 2017;41(7):1871–1881.
- Tanaka Y, Kato K, Notohara K, Hojo H, Ijiri R, Miyake T, et al. Frequent beta-catenin mutation and cytoplasmic/nuclear accumulation in pancreatic solid-pseudopapillary neoplasm. Cancer Res. 2001;61(23):8401–8404.
- Huang SC, Ng KF, Yeh TS, Chang HC, Su CY, Chen TC. Clinicopathological analysis of β-catenin and Axin-1 in solid pseudopapillary neoplasms of the pancreas. Ann Surg Oncol. 2012;19(Suppl 3):S438–S446.
- Sever R, Brugge JS. Signal transduction in cancer. Cold Spring Harb Perspect Med. 2015;5(4):a006098.
- Giroux S. Overcoming acquired resistance to kinase inhibition: The cases of EGFR, ALK and BRAF. Bioorg Med Chem Lett. 2013;23(2):394–401.
- Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: An evolving paradigm. Nat Rev Cancer. 2013;13(10):714–726.
- Lito P, Rosen N, Solit DB. Tumor adaptation and resistance to RAF inhibitors. Nat Med. 2013;19(11):1401–1409.
- Sanchez-Vega F, Mina M, Armenia J, Chatila WK, Luna A, La KC, et al. Oncogenic Signaling Pathways in The Cancer Genome Atlas. Cell. 2018;173(2):321–337.
- Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324(5933):1457–1461.
- Ougolkov AV, Bilim VN, Billadeau DD. Regulation of pancreatic tumor cell proliferation and chemoresistance by the histone methyltransferase enhancer of zeste homologue 2. Clin Cancer Res. 2008;14(21):6790–6796.
- Furukawa T. Impacts of activation of the mitogen-activated protein kinase pathway in pancreatic cancer. Front Oncol. 2015;5:23.
- Shen W, Tao GQ, Zhang Y, Cai B, Sun J, Tian ZQ. TGF-β in pancreatic cancer initiation and progression: two sides of the same coin. Cell Biosci. 2017;7:39.
- Guo N, Zhou QB, Chen RF, Zou SQ, Li ZH, Lin Q, et al. Diagnosis and surgical treatment of solid pseudopapillary neoplasm of the pancreas: analysis of 24 cases. Can J Surg. 2011;54(6):368–374.
- Yalçın B, Yağcı-Küpeli B, Ekinci S, Orhan D, Oğuz B, Varan A, et al. Solid pseudopapillary neoplasm of the pancreas in children: Hacettepe experience. ANZ J Surg. 2019;89(6):E236–E240.
- Jr Vollmer CM, Dixon E, Grant DR. Management of a solid pseudopapillary tumor of the pancreas with liver metastases. HPB (Oxford). 2003;5(4):264–267.
- Wu J, Tian X, Liu B, Li C, Hao C. Features and Treatment of Peritoneal Metastases from Solid Pseudopapillary Neoplasms of the Pancreas. Med Sci Monit. 2018;24:1449–1456.
- Cruz MA, Moutinho-Ribeiro P, Costa-Moreira P, Macedo G. Solid Pseudopapillary Neoplasm of the Pancreas: Unfolding an Intriguing Condition. GE Port J Gastroenterol. 2022;29(3):151–162.
- Tanoue K, Mataki Y, Kurahara H, Idichi T, Kawasaki Y, Yamasaki Y, et al. Multidisciplinary treatment of advanced or recurrent solid pseudopapillary neoplasm of the pancreas: three case reports. Surg Case Rep. 2022;8(1):7.
- Hilmi M, Bartholin L, Neuzillet C. Immune therapies in pancreatic ductal adenocarcinoma: Where are we now?. World J Gastroenterol. 2018;24(20):2137–2151.
- Brouwer TP, Vahrmeijer AL, de Miranda NFCC. Immunotherapy for pancreatic cancer: chasing the light at the end of the tunnel. Cell Oncol (Dordr). 2021;44(2):261–278.
Keywords
Frantz’s tumor; Solid-pseudopapillary neoplasm; Pancreatic carcinomas; Children; Metastasis; Management; Solid and cystic tumor; Exocrine pancreatic neoplasm
Cite this article
Eva G, Jana B, Irina L, Petrovich V. Pancreatic solid-pseudopapillary neoplasm in an adolescent girl presenting as an acute abdomen. Clin Case Rep J. 2022;3(4):1–6.
Copyright
© 2022 Jana Bernic. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-4.0).





