Giant Multiloculated Cystic Mesenteric Lymphangioma in a Child- Case Report and Review of Literature
Gudumac Eva;
* Jana Bernic;
Livsit Irina;
Virgil Petrovich;
-
Gudumac Eva: “Natalia Gheorghiu” Department of Pediatric Surgery, Orthopedics and Anesthesiology, “Nicolae Testemitanu” State University of Medicine and Pharmacy of the Republic of Moldova, Chisinau, Moldova.
-
* Jana Bernic: “Natalia Gheorghiu” Department of Pediatric Surgery, Orthopedics and Anesthesiology, “Nicolae Testemitanu” State University of Medicine and Pharmacy of the Republic of Moldova, Chisinau, Moldova.
-
Livsit Irina: “Natalia Gheorghiu” Department of Pediatric Surgery, Orthopedics and Anesthesiology, “Nicolae Testemitanu” State University of Medicine and Pharmacy of the Republic of Moldova, Chisinau, Moldova; “Natalia Gheorghiu” National Scientific and Practical Center for Pediatric Surgery, Research Institute for Mother and Child Health Care, Chisinau, Moldova.
-
Virgil Petrovich: “Natalia Gheorghiu” Department of Pediatric Surgery, Orthopedics and Anesthesiology, “Nicolae Testemitanu” State University of Medicine and Pharmacy of the Republic of Moldova, Chisinau, Moldova; “Natalia Gheorghiu” National Scientific and Practical Center for Pediatric Surgery, Research Institute for Mother and Child Health Care, Chisinau, Moldova.
-
Jun 17, 2022 |
-
Volume: 3 |
-
Issue: 5 |
-
Views: 2913 |
-
Downloads: 2018 |
Abstract
A clinical case of the lymphangioma in a child and the role of both anamnestic, clinical, and paraclinical data, as well as histopathological data in the differentiation of intra-abdominal tumors and the prognosis, are reported.
Introduction
Recent studies show that lymphangiomas account for 5%–9% of all benign tumors in children [1]. In 8%–15% of cases, lymphangiomas are internally located. Abdominal lymphangioma is an extremely rare benign anomaly. Of all intra-abdominal lymphangiomas, 70% are located in the mesentery, and the rest in the gastric, duodenal, small intestine, colon, pancreas, spleen, liver, retroperitoneal [2]. Mesenteric lymphangiomas may be present in any part of the mesentery and mesocolon, but most commonly in the ileal mesentery and right mesocolon (approximately 60% of the ileal portion 40% of the ascending mesocolon). Considering the rarity of abdominal lymphangiomas and the difficulties of differential diagnosis of this pathology, each new case is of undoubted practical interest.
Case Presentation
We present the description of the clinical observation of a 10-year-old boy. He presents urgently to the district hospital with abdominal pain. Subsequently, the child was transferred to “Natalia Gheorghiu” National Scientific and Practical Center for Pediatric Surgery, Research Institute for Mother and Child Health Care (Chisinau, Moldova). The symptoms started three hours before the presentation in our pediatric surgery service. The patient did not have a previous pathology and was not under medical treatment.
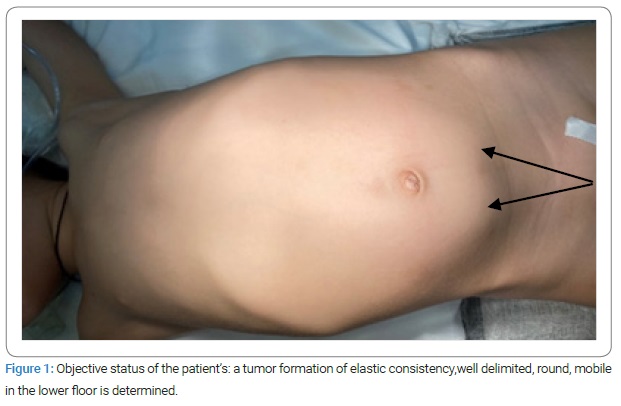
The objective at admission: The patient is conscious, cooperating, afebrile, the general condition of medium severity, presents accusations of periodic abdominal pain, mainly in the lower-left side, and the patient is hemodynamically stable. The abdomen is slightly distended, asymmetrical, without muscular defense, and sensitive to palpation on the lower floor, where a tumor formation of elastic consistency, well delimited, round, and mobile, is determined. The peritoneal symptoms were negative. The child has no urinary or digestive tract disorders (Figure 1).
Laboratory examination at admissionrevealed: Blood group B (III), Rh positive; hemoglobin – 119 g/l; erythrocytes - 3,9.1012/l; hematocrit - 0.35 l/l; leukocytes - 8,7.109/l; unsegmented – 12%; segmented – 40%; eosinophils – 6%; lymphocytes – 46%; monocyte – 6%; ESR – 6; platelets -454.109/l; clotting time – 4min.
Blood biochemistry revealed: total protein - 75 g/l; total bilirubin and its fractions - 7.3-0-7.3 µM/l; ALT – 46 U/l; AST –30 U/l; calcium - 2,85 mM/l; sodium – 139 mM/l; potasium - 2.32 mM/l; chlorine - 94.6 mM/l; Fe - 11.4 µM/l; Mg - 0.84 mM/l; prothrombin - 92%; fibrinogen - 2.2 g/l.
Urinalysis demonstrated: color – yellow, transparent, acid reaction, protein - negative, flat epithelium 2–3 in the field of view, leukocytes 2–4 in the field of view, salts - slightly oxalate.
Thus, emergency biological markers reveal moderate anemia and normal liver and kidney function.
Abdominal ultrasound reveals a cystic formation above the bladder measuring 127 mm x 51 mm, with liquid content and multiple septa.
Computed tomography performed urgently in the native and arterial phase shows a multilocular cystic formation, with a density of 12 UH–17 UH, dimensions 15 cm x 12 cm x 8.3 cm, which moves the small intestine anteriorly and laterally, and the bladder anteriorly, without other pathological aspects. This formation does not capture the contrast substance; while is thin 1.3 mm–1.5 mm capsule intensively captures the contrast substance, the contour is bumpy and clear. Free intra-abdominal fluid is not evident. Sufficient contrast of the abdominal aorta and its branches, bifurcation, as well as the vena cava, portal vein, and their afferents, is determined. The paracaval and paraaortic lymph nodes are not enlarged—conclusion: intra-abdominal multilocular cystic formation, possibly located in the meso. Following the investigations, an abdominal cystic tumor was diagnosed. The most appropriate therapeutic attitude to solve this case was surgery, which was performed under oro-tracheal anesthesia and mechanical ventilation.
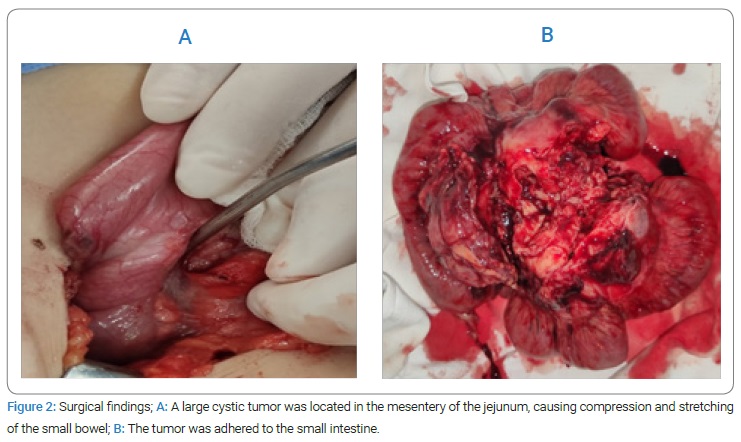
After a preoperative preparation in these conditions, surgery was performed - lower median laparotomy. When the abdominal cavity was opened, 250 ml of colorless, odorless serum fluid was removed. At the revision of the abdominal organs at a distance of 30 cm–35 cm from the Treitz ligament, a large, multicameral cystic tumor was detected, which was located between the sheets of the mesentery of the small intestine with the dimensions of 8 cm x 6 cm x 5 cm. The omentum was attached to the given cystic formation, which was later partially resected. The cyst membrane was translucent and thin. The small intestine was resected with the multicystic formation of the meso. The small intestine involved in the process was about 9 cm–10 cm long, being slightly inflamed and hyperemic.
Restoration of intestinal transit was performed by applying the lateral-lateral ileo-ileal anastomosis. Sol. Novocaine 0.25% 10 ml was introduced into the meso. At the closure of the peritoneal cavity, 200 ml of 5% solution of Aminocapronic Acid + 3 ampoules of Thrombin (each having at least 125 IU of coagulation activity) was introduced. The peritoneal cavity was drained through a separate counter aperture in the right ileal region with a glove lamella. The anatomical plans were redone, and an aseptic dressing was applied (Figure 2).
Histopathological examination confirmed the diagnosis of multicameral cavernous cystic lymphangioma of the meso, with partial involvement of the small intestinal wall (ileum) and lymphohistiocytic infiltration into the foci.
Postoperative conservative treatment included:
- Nutritional support, parenteral nutrition for a period of 3 days.
- Administration of infusion fluids until the resumption of optimal oral hydration.
- Intravenous antibiotic therapy for at least three days, followed by oral antibiotic therapy.
- Intravenous proton pump inhibitors, then orally for at least 30 days.
- Analgesics, sedatives, intestinal prokinetics, antihypoxic drugs.
The gastric tube was removed 12 hours postoperatively. Oral rehydration with clear liquids (tea, water, soups, compote) began at 72 hours, followed by skim dairy products.
Then, enteral feeding was resumed in the presence of transit for gas and feces.
The patient was discharged on the eighth day postoperatively in the conditions in which he was hemodynamically stable, afebrile, had optimal digestive tolerance, intestinal transit present, and postoperative wound healing.
Discussion
Mesenteric lymphangiomas are undoubtedly a common condition in children. The continuous development of medical techniques (CT, MRI, Angiography) has increased the incidence of this disease, which remains rare. The etiology of lymphomas is uncertain, not excluded, due to a congenital anomaly of the lymphatic system that causes the seizure of lymphatic tissue during embryological development [3–6].
This theory explains the appearance of lymphangiomas, especially in children. In addition, some authors suggest that abdominal trauma, lymphatic canal obstruction, inflammatory processes, tuberculosis, history of surgery, or radiation therapy may lead to these pathology [7,8].
Macroscopically, lymphangiomas have a spheroidal or ovoid appearance of variable dimensions that can reach a diameter of 2 mm–3 mm and up to 25 cm or even more. The outer surface is smooth, rarely bumpy, and whitish in color. They are located in the place of the accumulation of lymph nodes. The cavity is usually unique, sometimes septate, by incomplete septa. The bottom surface is smooth and glossy. The content of lymphangioma, more often, is clear, transparent, colorless, flowing, homogeneous, low-density, and does not coagulate but contains suspension salts, glucose, urea, protein substances (albumin, fibrin), and figurative elements of the blood. Sometimes the cyst’s contents can be yellow-green (cystic lymph nodes, hilarious due to suspended lipids), yellowish, greasy, bloody, brown, or serocitrin [9].
Microscopically, the cystic lymphangioma wall has three layers: An internal endothelial layer, usually discontinuous. This layer is more degraded when the cyst the older, and in some cases, it is missing. Outside this endothelium is a middle layer consisting of condensation of loose, vascularized connective tissue, in which there are few elastic elements and sometimes smooth muscle fibers. Numerous lymphoid elements are constantly observed and grouped together, forming true follicles that constitute the characteristic element. Lymphatic cavities and gaps with leukocyte and gigantocellular elements of the microphage type often appear here. The outer layer consists of adult connective tissue, poorly vascularized, with peritoneal dependence, which forms the pedicle surrounded by fatty tissue [10].
Lymphangiomas are characterized by slow growth, asymptomatic evolution, infiltrative growth, and often recurrence. Malignancy data are not reported in the literature.
The clinic may be asymptomatic, either with an enlarged abdomen in volume and/or the presence of a palpable formation or with an acute clinical picture of the abdomen. For the first two variants, the asymmetry of the abdomen is characteristic; a hard, painless, weakly mobile formation is determined by palpation. For the last variant of the clinic, the presence of abdominal pain is characteristic, which appears only in case of association of complications, which gives the clinic an acute abdomen [11]. Laboratory data show no signs of inflammation. Complications that may occur during the evolution of lymphangioma are intestinal obstruction, digestive hemorrhage by rupture of the lymphangioma in the intestinal lumen or ulceration of the lymphangioma, intestinal infarction, infection of the lymphangioma [12]. Abdominal ultrasound confirms the presence of a multicameral, hypoechoic, or anechoic formation. “Empty” abdominal radiographs show the existence of a liquid mass that can push the intestinal loops. Computer tomography is required in more difficult cases.
The differential diagnosis will be made with: simple lymphatic mesenteric cysts; pancreatic pseudocyst; hydatid cyst; local ascites (malignant or infectious - for example, in tuberculosis); peritoneal inclusion cysts; mesenteric cystic panniculitis (sclerosing mesentery) [13]. Treating complete surgical excision would be the ideal treatment method in lymphangiomas. However, in some patients, lymphangiomas often grow aggressively and reach an enormous size. Therefore, some patients require a bowel resection for complete cyst excision (incorporation of blood vessels or immediate vicinity with them).
Conclusion
Mesenteric lymphangiomas are rare clinical features in children and are mostly congenital but have gained increasing incidence in recent years. This is relative due to ultrasound, computed tomography, and MRI scans. The clinical manifestations of mesenteric lymphangiomas are nonspecific, and despite the methods of diagnosis and differential diagnosis, they are still a condition that causes complications and the association with disabilities. The vast majority of these formations evolve asymptomatically, being accidentally discovered. The dominant objective sign is the palpation of the tumor, which depends on the size of the formation, the clinical-evolutionary phase, the degree of modification of the abdominal wall, the intestinal loops, and other organs. Among the most useful paraclinical explorations are the imaging ones such as abdominal ultrasound, computed tomography, and MRI, as well as isotope explorations and histopathological ones. The treatment of choice in mesenteric lymphangiomas is surgical, and the volume of surgery depends on the size, location of the lesion in the mesentery, and the nature of complications. Surgical treatments in the early stages, until the association of complications, radically improve the prognosis. Histological examination of the piece is mandatory and confirms the diagnosis of lymphangioma. Late examinations will be carried out between 6 months and three years, with the aim of verifying the stability and effectiveness of the result over time if no recurrences have occurred.
In conclusion, it is emphasized that improvements are needed in making the diagnosis. However, as a surgical feature, extensive resections can be performed without risk to the patient and with further benefits.
Acknowledgments
This study was supported by the State Program of the Republic of Moldova (research grant no. 20.080009.5007.8007.06).
Authors’ contributions: Gudumac Eva, Bernic Jana, and Livsit Irina participated in the whole diagnostic and treatment process of the described patients. Gudumac Eva and Bernic Jana conceived the concept of the study. Bernic Jana and Livsit Irina collected the data. Virgil Petrovich participates in the histopathological examination. Gudumac Eva wrote the manuscript, and all authors contributed to revisions. Finally, all authors read and approved the final manuscript.
Ethical Approval Consent: Written informed consent was obtained from the patient legal guardian for publication of this case report and any accompanying images, both orally and in writing, in accordance with the principles of the Helsinki Treaty with subsequent revisions and additions.
Ethics Committee Approval
The biological specimens were collected according to the current research principles, approved by the Research Ethics Committee of NicolaeTestemitanu State University of Medicine and Pharmacy (positive opinion dated February 25, 2021, report No. 6).
Conflict of Interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Informed consent was obtained for this publication.
References
- Clement C, Snoekx R, Ceulemans P, Wyn I, Matheï J. An acute presentation of pediatric mesenteric lymphangioma: a case report and literature overview. Acta Chir Belg. 2018;118(5):331–335.
- Karkera PJ, Sandlas GR, Ranjan RR, Kesan K, Gupta AR, Gupta RK, et al. Intra-abdominal cystic lymphangiomas in children: A case series. Arch Int Surg. 2012;2(2):91–95.
- Hancock BJ, St-Vil D, Luks FI, Di Lorenzo M, Blanchard H. Complications of lymphangiomas in children. J Pediatr Surg. 1992;27(2):220–224.
- Paal E, Thompson LD, Heffess CS. A clinicopathologic and immunohistochemical study of ten pancreatic lymphangiomas and a review of the literature. Cancer. 1998;82(11):2150–2158.
- Abe H, Kubota K, Noie T, Bandai Y, Makuuchi M. Cystic lymphangioma of the pancreas: a case report with special reference to embryological development. Am J Gastroenterol. 1997;92(9):1566–1567.
- Fernandez-Vega I. Cystic lymphangioma: an uncommon cause of pediatric abdominal pain. Int J Pathol Clin Res. 2017;3:53.
- Losanoff JE, Richman BW, El-Sherif A, Rider KD, Jones JW. Mesenteric cystic lymphangioma. J Am Coll Surg. 2003;196(4):598–603.
- Wani I. Mesenteric lymphangioma in adult: a case series with a review of the literature. Dig Dis Sci. 2009;54(12):2758–2762.
- Alqahtani A, Nguyen LT, Flageole H, Shaw K, Laberge JM. 25 years’ experience with lymphangiomas in children. J Pediatr Surg. 1999;34(7):1164–1168.
- Ignjatovic I, Milosavljevic V, Tadic B, Grubor N, Matic S. Lymphangioma of the Small Intestine Case Report and Review of the Literature. Serbian Journal of Experimental and Clinical Research. 2019;20(4):357–360.
- Kusuma P, Putra MDP, Suwardi S. Mesenteric Cystic Lymphangioma in Pediatric Patient: A Rare Intra-Abdominal Tumor Management in Rural Country Case Report. Maced J Med Sci. 2021;9(C):84–88.
- Mahmoudi A, Rami M, Khattala K, El Madi A, Bouabdallah Y. Huge omental lymphangioma with haemorrhage in children: case report. Pan Afr Med J. 2020;35:20.
- Abdelkader A, Hunt B, Hartley CP, Panarelli NC, Giorgadze T. Cystic Lesions of the Pancreas: Differential Diagnosis and Cytologic-Histologic Correlation. Arch Pathol Lab Med. 2020;144(1):47–61.
Keywords
Cysticmezenteric lymphangioma; Abdomen; Children
Cite this article
Eva G, Jana B, Irina L, Petrovich V. Giant Multiloculated Cystic Mesenteric Lymphangioma in a Child- case report and review of literature. Clin Case Rep J. 2022;3(5):1–5.
Copyright
© 2022 Jana Bernic. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-4.0).


