Safety of Re-Vaccination after Developing Urticaria at the First Dose of Chadox1 nCov-19
Kanokkarn Pinyopornpanish;
Narapong Yotinnoratham;
Nattakirana Thongdee;
Nizchapa Dechapaphapitak;
Pansa Iamrahong;
Chulapha Ruangwattanachok;
Wannada Laisuan;
* Apinya Chungcharoenpanich;
-
Kanokkarn Pinyopornpanish: Division of Allergy, Immunology and Rheumatology, Ramathibodi Hospital, Mahidol University, Bangkok 10400, Thailand.
-
Narapong Yotinnoratham: Division of Allergy, Immunology and Rheumatology, Ramathibodi Hospital, Mahidol University, Bangkok 10400, Thailand.
-
Nattakirana Thongdee: Division of Allergy, Immunology and Rheumatology, Ramathibodi Hospital, Mahidol University, Bangkok 10400, Thailand.
-
Nizchapa Dechapaphapitak: Division of Allergy, Immunology and Rheumatology, Ramathibodi Hospital, Mahidol University, Bangkok 10400, Thailand.
-
Pansa Iamrahong: Division of Pharmacy, Ramathibodi Hospital, Mahildol University, Bangkok 10400, Thailand.
-
Chulapha Ruangwattanachok: Division of Pharmacy, Ramathibodi Hospital, Mahildol University, Bangkok 10400, Thailand.
-
Wannada Laisuan: Division of Allergy, Immunology and Rheumatology, Ramathibodi Hospital, Mahidol University, Bangkok 10400, Thailand.
-
* Apinya Chungcharoenpanich: Division of Allergy, Immunology and Rheumatology, Ramathibodi Hospital, Mahidol University, Bangkok 10400, Thailand.
Abstract
The World Health Organization (WHO) approved the AstraZeneca/Oxford COVID-19 vaccine (ChAdOx CoV-19) for emergency use in February 2021. The WHO’s Strategy Advisory Group of Experts on Immunization recommended use for all age groups 18 and above. However, after the ChAdOx CoV-19 vaccine rollout, allergic reaction following ChAdOx CoV-19 vaccination was reported, causing vaccine hesitancy. Although a heterologous prime-boost strategy could be used in those who have had such an allergic reaction, the safety and vaccine efficacy of such a strategy remains controversial. Instead, it may be safer for such patients to be re-vaccinated with the same vaccine associated with allergic reactions. Thus, we report seven cases of successful safe re-vaccination in individuals who had previously experienced immediate/delayed urticaria/angioedema following vaccination with the ChAdOx CoV-19 vaccine.
Introduction
Coronavirus disease 2019 (COVID-19) vaccination is one of the strategies used to mitigate the disease severity during the worldwide pandemic. Vaccination is encouraged in all populations since it is proven effective in preventing disease. However, some adverse events following immunization are of concern, including allergic reaction [1,2] – rash, burning skin sensation, and red welts on the face and lips. In addition, vaccine hypersensitivity can harm the vaccine and affect the decision to receive the next vaccination dose.
The AstraZeneca/Oxford COVID-19 (ChAdOx CoV-19) vaccine is a viral vector vaccine that was approved by the World Health Organization (WHO) for emergency use in February 2021 [3]. After vaccine rollouts, rash, angioedema, and anaphylaxis associated with COVID-19 vaccination were reported [4–6]. The unknown mechanism is believed to be due to either IgE or non-IgE mediated hypersensitivity. Due to the situation in Thailand, the ChAdOx CoV-19 vaccine was the main vaccine available, and mRNA vaccines, along with most other platforms, were unavailable. Thus, re-vaccination was considered in case of a negative skin test for polysorbate.
Polysorbate 80, a potential allergen, is an excipient in the ChAdOx CoV-19 vaccine [7]. A previous study found that polysorbate 80 could cause severe nonimmunological anaphylaxis [8] and cause type IV hypersensitivity [9]. Also, cross-reactivity between Polyethylene Glycol (PEG) and polysorbate has been reported [10]. It has been suggested that patients with a history of severe allergic reactions to polysorbate should avoid vaccination with the ChAdOx CoV-19 vaccine. The mRNA vaccine may be a preferable option [11]. However, there is a lack of data on the vaccine efficacy of using a heterologous prime-boost strategy. Vaccine types are limited in some countries with governmental strategic plans for mass vaccination. Allergology work-up by allergists and sharing decision-making with patients should be performed. According to the ENDA/EAACI position paper’s recommendation, re-vaccination may be considered in individuals with a negative skin test to the excipients and those assessed as medium risk [12,13].
Pre-medication with an antihistamine remains a controversial issue because it may mask the initial symptom of systemic reactions, causing delayed diagnosis. However, pre-medication may be considered in individuals with a history of mild allergic reactions [13].
Thus, we describe a case series of the vaccination outcomes in individuals who experienced urticaria/angioedema following vaccination with the ChAdOx CoV-19 vaccine, the subsequent skin testing results, and the re-vaccination outcomes.
Materials and Methods
A prospective study was conducted in the Allergy Unit of Ramathibodi Hospital, a tertiary university hospital of Mahidol University, Bangkok, Thailand. Ethical approval was granted by the Ramathibodi HospitalInstitutional Review Board (MURA2021/641). Inclusion criteria were individuals who had urticaria/angioedema following vaccination with the ChAdOx CoV-19 vaccine at any time of reaction onset after vaccination between July 1, 2021, and September 30, 2021. Informed consent was obtained from the included patients. Individuals who denied signing the consent form or were pregnant mothers were excluded. The study was performed in accordance with the Declaration of Helsinki.
Patients’ demographic data, characteristics of reactions, and underlying diseases were collected. The reaction was classified by time-to-onset the reaction. An immediate reaction was defined as occurring within two hours of vaccination [14], and a delayed reaction was defined as occurring more than two hours after vaccination. Allergology skin testing of polyethylene glycol 1500 (PEG 1500), polyethylene glycol 4000 (PEG 4000), and polysorbate 80 were performed. The reference concentration was applied according to the method of a previous study [13]. Full-dose re-vaccination with/without antihistamine premedication was performed. The outcomes of re-vaccinations were recorded.
Results
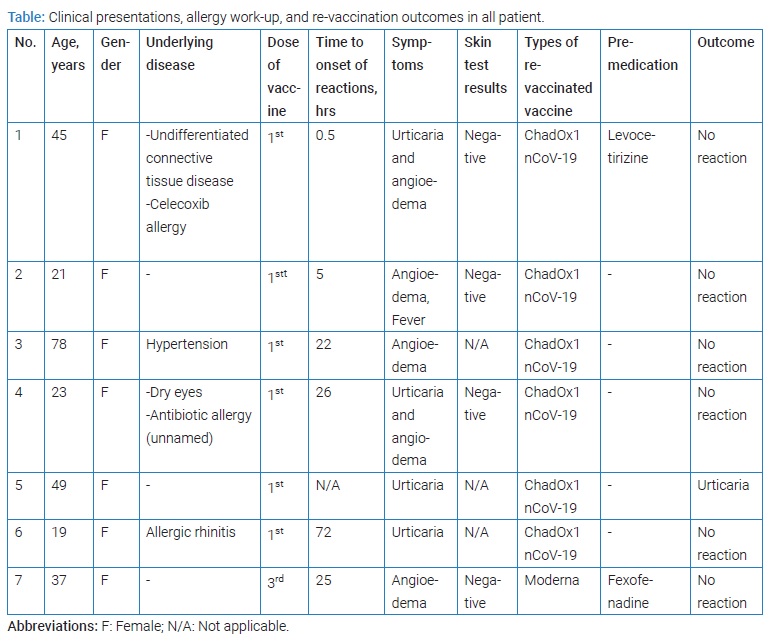
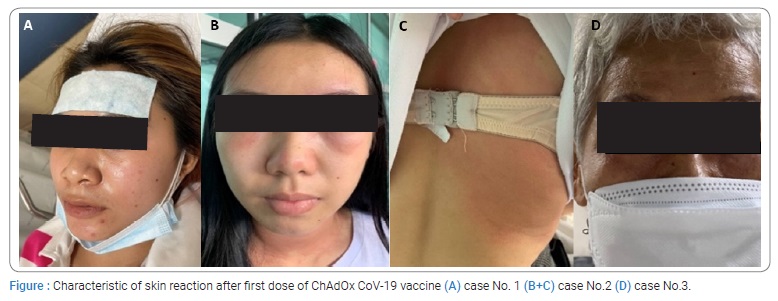
The characteristics of the seven patients, all of whom were female and had urticaria/angioedema, are shown in (Table). Five patients developed delayed onset urticaria/angioedema reactions following vaccination with the ChAdOx CoV-19 vaccine. In contrast, one participant developed urticaria within 30 minutes following vaccination, and another participant could not identify the onset of reaction. The skin lesions are shown in (Figure). In addition, two patients experienced the urticaria and angioedema while three participants had the angioedema reaction, and two participants had an urticaria reaction. The time-to-resolution of these reactions ranged from one hour to five days.
Four patients with negative results performed skin tests for PEG and polysorbate 80 (Table). In addition, antihistamine premedication was applied in two patients before full-dose re-vaccination with the ChAdOx CoV-19 vaccine.
All participants safely completed the vaccination series; only one participant had a mild reaction, which was urticaria, at 40 hours following re-vaccination.
Discussion
The mechanism of allergic reactions is still unclear [15]. In the present study, the evidence of IgE-mediated hypersensitivity to the vaccine’s excipients could not be confirmed, and skin tests for PEG and polysorbate were negative. However, the basophil activation test was not performed. In a prior study, 2 of 25 patients with allergic reactions had positive evidence of IgE-mediated hypersensitivity, implying that non-IgE mediated could be the main mechanism of hypersensitivity reactions following immunization [16]. In addition, previous studies have shown that polysorbate has the potential to induce urticaria and anaphylactoid reactions [8,17,18].
Individuals who have had a negative skin test can probably be re-vaccinated safely. In the present study, we successfully administered full-dose re-vaccination to all seven individuals who had experienced only urticaria/angioedema at any onset following vaccination with the AstraZeneca/Oxford vaccine. None of these patients had a reaction within 24 hours after vaccination. However, one of the seven patients had urticaria at 40 hours following vaccination. This delayed urticarial rash was resolved by oral antihistamine treatment. This descriptive case series suggests that individuals who had only urticaria/angioedema following vaccination with the AstraZeneca/Oxford vaccine are not contraindicated to complete a vaccine series. Furthermore, due to the skin test result, this study might confirm that the mechanism of urticaria is not IgE-mediated hypersensitivity.
Premedication with the antihistamine may be considered in patients suspected of mild allergic reaction to the vaccine [13]. In the present study, we administered premedication of antihistamine to two subjects. Daily use of the antihistamine should be suggested for an individual who has chronic spontaneous urticaria, and the dosage should be adjusted to control the urticarial symptom. However, antihistamine premedication should be avoided in patients suspected of severe allergic reactions because it may mask the systemic reaction, causing delayed diagnosis [19,20]. Following EAACI 2022 guidelines, a patient with delayed urticaria might not need to have a skin test, and premedication might be considered.
Our study has the limitation of a small sample size because of the change in relevant government policy concerning the recommended vaccine guidelines and the arrival of mRNA vaccines, which precluded further recruitment under our eligibility criteria.
Conclusion
Re-vaccination could be performed safely after diagnostic work-up in individuals who had only urticaria/angioedema at any time of onset following vaccination with the ChAdOx CoV-19 vaccine? Premedication with an antihistamine may be considered in individuals with mild allergic reactions. Further, more extensive study is needed.
Author Contributions
All authors contributed substantially to the conception and design, acquisition of data, or analysis and interpretation of data. All authors took part in drafting the article or revising it critically for important intellectual content. All authors have agreed to submit to the current journal, have given approval for the final version to be published, and have agreed to be accountable for all aspects of the work.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111.
- Menni C, Klaser K, May A, Polidori L, Capdevila J, Louca P, et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect Dis. 2021;21(7):939–949.
- AstraZeneca. COVID-19 vaccine authorised for emergency supply in the UK [Internet]. UK: AstraZeneca; 2020.
- Kelso JM. Anaphylactic reactions to novel mRNA SARS-CoV-2/COVID-19 vaccines. Vaccine. 2021;39(6):865–867.
- FDA. Reports of suspected adverse reaction to COVID-19 vaccines. Philippines: Food and drug administration philippines; 2021.
- MHRA. Coronavirus vaccine-weekly summary of Yellow card reporting. UK: Medicines and Healthcare products Regulatory Agency; 2021.
- Cabanillas B, Akdis CA, Novak N. Allergic reactions to the first COVID-19 vaccine: A potential role of polyethylene glycol? Allergy. 2021;76(6):1617–1618.
- Coors EA, Seybold H, Merk HF, Mahler V. Polysorbate 80 in medical products and nonimmunologic anaphylactoid reactions. Ann Allergy Asthma Immunol. 2005;95(6):593–599.
- Lucente P, Iorizzo M, Pazzaglia M. Contact sensitivity to Tween 80 in a child. Contact Dermatitis. 2000;43(3):172.
- Jr. Stone CA, Liu Y, Relling MV, Krantz MS, Pratt AL, Abreo A, et al. Immediate hypersensitivity to polyethylene glycols and polysorbates: more common than we have recognized. J Allergy Clin Immunol Pract. 2019;7:1533–1540.e8.
- Glover RE, Urquhart R, Lukawska J, Blumenthal KG. Vaccinating against covid-19 in people who report allergies. BMJ. 2021;372:n120.
- Wolfson AR, Robinson LB, Li L, McMahon AE, Cogan AS, Fu X, et al. First-dose mrna COVID-19 vaccine allergic reactions: limited role for excipient skin testing. J Allergy Clin Immunol Pract. 2021;9(9):3308–3320.e3.
- Banerji A, Wickner PG, Saff R, Jr. Stone CA, Robinson LB, Long AA, et al. mRNA vaccines to prevent COVID-19 disease and reported allergic reactions: current evidence and suggested approach. J Allergy Clin Immunol Pract. 2021;9(4):1423–1437.
- Barbaud A, Garvey LH, Arcolaci A, Brockow K, Mori F, Mayorga C, et al. Allergies and COVID-19 vaccines: An ENDA/EAACI Position paper. Allergy. 2022.
- Sun Q, Fathy R, McMahon DE, Freeman EE. COVID-19 vaccines and the skin: the landscape of cutaneous vaccine reactions worldwide. Dermatol Clin. 2021;39(4):653–673.
- Rasmussen TH, Mortz CG, Georgsen TK, Rasmussen HM, Kjaer HF, Bindslev-Jensen C. Patients with suspected allergic reactions to COVID-19 vaccines can be safely revaccinated after diagnostic work-up. Clin Transl Allergy. 2021;11(5):e12044.
- Perez-Perez L, Garcia-Gavin J, Pineiro B, Zulaica A. Biologic-induced urticaria due to polysorbate 80: usefulness of prick test. Br J Dermatol. 2011;164(5):1119–1120.
- Kato M, Oiso N, Uchida S, Yanagihara S, Sano H, Tohda Y, et al. Biologic-induced urticaria due to polysorbate 20. J Dermatol. 2019;46(7):e230–e232.
- Turner PJ, Ansotegui IJ, Campbell DE, Cardona V, Ebisawa M, El-Gamal Y, et al. COVID-19 vaccine-associated anaphylaxis: a statement of the world allergy organization anaphylaxis committee. World Allergy Organ J. 2021;14(2):100517.
- Public Health England. COVID-19: the green book (chapter 14) [Internet]. England: Public Health England; 2021.
Keywords
Allergy; COVID-19 vaccine; Vaccine hypersensitivity; Re-vaccination; Urticaria
Cite this article
Pinyopornpanish K, Yotinnoratham N, Thongdee N, Dechapaphapitak N, Iamrahong P, Ruangwattanachok C, et al. Safety of re-vaccination after developing urticaria at the first dose of ChAdOx1 nCoV-19. Clin Case Rep J. 2022;3(5):1–4.
Copyright
© 2022 Apinya Chungcharoenpanich. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-4.0).


