The Association between Vasospasm and Serum Concentrations of Aldehyde Dehydrogenase 2 in Patients with Variant Angina
Kyu Tae Park;
Jun Seok;
Kyung-Soon Hong;
* Hyun Hee Choi;
-
Kyu Tae Park: Department of Internal Medicine, Division of Cardiology, Hallym University College of Medicine, Chuncheon, South Korea.
-
Jun Seok: Division of Infectious Disease Control, Health and Welfare Bureau, Jeollanam-do Provincial Government, South Korea.
-
Kyung-Soon Hong: Department of Internal Medicine, Division of Cardiology, Hallym University College of Medicine, Chuncheon, South Korea.
-
* Hyun Hee Choi: Department of Internal Medicine, Division of Cardiology, Hallym University College of Medicine, Chuncheon, South Korea.
Abstract
Background: Vasospastic angina is caused by vasospasm of the coronary arteries. Aldehyde dehydrogenase 2 (ALDH2) is involved in nitroglycerin metabolism and reduces oxidative stress, thereby suppressing vasospasm. However, it is unclear whether a change in the expression level of human ALDH2 in the blood correlates with the regulation of vasospastic angina.
Methods: This was a retrospective study of patients who had undergone coronary angiography (CAG) owing to angina; 2,326 patients underwent CAG, and 195 underwent an intracoronary ergonovine provocation test because of suspected vasospastic angina symptoms and no significant stenosis on baseline CAG.
Results: Vasospastic angina was present in 39% of the subjects, as determined by the ergonovine provocation test. The difference in ALDH2 concentration before and after the provocation test was much larger in the positive provocation group (−10.002 ± 4.53, p = 0.03 vs. −4.486 ± 3.469, p = 0.199).
Conclusions: The concentration of human ALDH2 in the blood is associated with vasospastic angina.
Introduction
In 1959, Prinzmetal et al. described 32 cases of a variant form of angina characterized by rest pain associated with transient ST-segment elevation. The angina episodes often recurred, frequently waking the patient [1]. In addition, coronary spasm has been shown to play a key role in several cases of variant angina [2,3].
The prevalence of variant angina is apparently higher in East Asian populations than in their Western counterparts [4,5]. For example, in a recent study of 2,129 patients in the VA-Korea (Vasospastic Angina in Korea) registry, the positivity rate of ergonovine provocation was 21.3% [6].
The pathogenesis of coronary spasms is multifactorial and heterogeneous among different populations. Several studies have reported that the parasympathetic nervous system may play a role in the pathogenesis of coronary spasms and coronary vascular smooth muscle hyperactivity [7,8]. Endothelial cell dysfunction and deficiency in nitric oxide activity also contribute to the pathogenesis of coronary spasms [3]. Environmental factors such as smoking, insulin resistance, and alcohol consumption might also be considered pathogenic contributors [5,9].
East Asian variants of the aldehyde dehydrogenase 2 (ALDH2) ALDH2*2 genotypes are associated with vasospastic angina [10]. Two main variants of ALDH2 in East Asian populations; arise from replacing glutamate (Glu) at position 487 with lysine (Lys). The Glu allele (also designated ALDH2*1) encodes a protein with normal catalytic activity, whereas the Lys allele (ALDH2*2) encodes an inactive protein. As a result, Lys/Lys homozygotes have no detectable ALDH2 activity. Furthermore, because the Lys allele acts semi-dominantly, ALDH2 Lys/Glu heterozygotes have far less than half the ALDH2 activity of Glu/Glu homozygotes; in fact, the reduction in ALDH2 activity in heterozygotes is more than 100-fold [11]. Genetic variants of ALDH2 are commonly found in as many as 35%–45% of East Asians (Chinese, Japanese, Koreans, and Taiwanese) but are rare or absent in other ethnic populations worldwide [12,13].
Human ALDH2, one of 19 functional ALDH genes, has the chromosomal locus 12q24 and encodes a 517-amino acid polypeptide with mitochondrial subcellular localization [11,14].
ALDH2 plays a key role in oxidizing endogenous aldehyde products that arise from lipid peroxidation under oxidative stress, such as 4-hydroxy-2-nonenal (4-HNE) and malondialdehyde (MDA), as well as environmental aldehydes [15,16].
The cardiac protective effects of mitochondrial ALDH2 during cardiac ischemia and reperfusion injury in models of ethanol-mediated cardiomyopathy and heart failure have been demonstrated by using a variety of ALDH2 transgenic mice [17,18]. In summary, the positive role of ALDH2 in reducing oxidative stress by detoxifying reactive aldehydes may contribute to cardiac protection.
Moreover, ALDH2 is involved in the metabolism of nitroglycerin when it is used as a vasodilator. ALDH2 plays an essential role in the bioconversion of nitroglycerin to nitric oxide to achieve its vasodilating effects [4,19]. Therefore, ALDH2 is expected to play an important role in vasospastic angina. However, little active research has been undertaken on the role of ALDH2 in disorder.
To date, only studies on the genetic deficiency of ALDH2 have been undertaken, and the quantitative investigation of human ALDH2 expression levels in the blood has not yet been carried out. Therefore, in this study, we investigated whether a change in the human ALDH2 expression level in the blood plasma correlates with the regulation of vasospastic angina.
Our aims were:
- To compare and analyze the characteristics of the positive and negative cases of vasospastic angina revealed by intracoronary ergonovine provocation tests.
- To determine the blood plasma human ALDH2 concentration in vasospastic angina cases.
- To investigate the correlation between ALDH2 and vasospastic angina.
Materials and Methods
Study design and participants: This was a retrospective study of patients who underwent coronary angiography (CAG) in Chuncheon Sacred Heart Hospital between June 2011 and October 2014. Of those patients, 2,326 underwent CAG, and 203 underwent an intracoronary ergonovine provocation test because of suspected vasospastic angina symptoms and no significant stenosis on baseline CAG. One hundred and eighty-five patients for whom medical history and blood sample diagnosis information were available were included as study subjects (Figure 1).
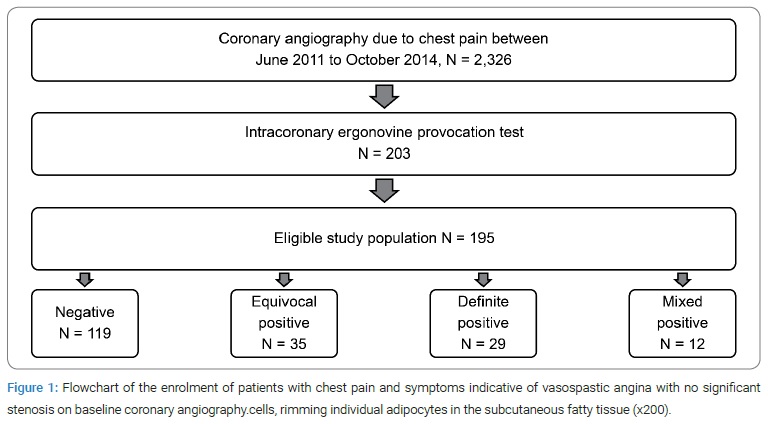
The included subjects completed a survey and submitted a blood sample that met the diagnostic criteria for coronary artery disease. In addition, arterial blood samples were collected via a trans-radial catheter to compare ALDH2 concentrations before and after the ergonovine provocation test. The plasma was extracted from the blood samples by centrifugation and was stored at −72°C. The patient records were subsequently reviewed to investigate vasospastic angina’s prevalence and relevant risk factors.
Ethics statement: The study protocol was approved by the Cardiology Ethics Committee, the institutional review board of Chuncheon Sacred Heart Hospital (IRB No. 2017-36). The IRB confirmed informed consent.
CAG and provocation test for vasospastic angina: Diagnostic CAG was performed via the trans-radial approach. After confirming the absence of significant coronary artery stenosis, incremental doses of ergonovine were infused into the left coronary artery. Ergonovine was then injected in the following order: E1 (20 µg), E2 (40 µg), and E3 (60 µg), with 2-minute intervals between consecutive doses. CAG was performed 2 minutes after completion of the injection. In the event of an ischemic change on the electrocardiogram (ECG) or a chest symptom, angiography was performed at the time of onset. In the event of a negative provocation test result, a right coronary provocation test was performed 5 minutes later. Coronary spasm during the ergonovine provocation test was defined as “transient, total, or subtotal occlusion (stenosis of > 90%) of a coronary artery with signs of myocardial ischemia (angina pain and ischemic ST changes)” [20]. Once coronary spasm had been induced, a sufficient dose of nitroglycerin was administered to each coronary artery, and angiography was performed while the coronary artery was maximally dilated. Aortic pressure, O2 saturation, and ECG signals were recorded during the procedure.
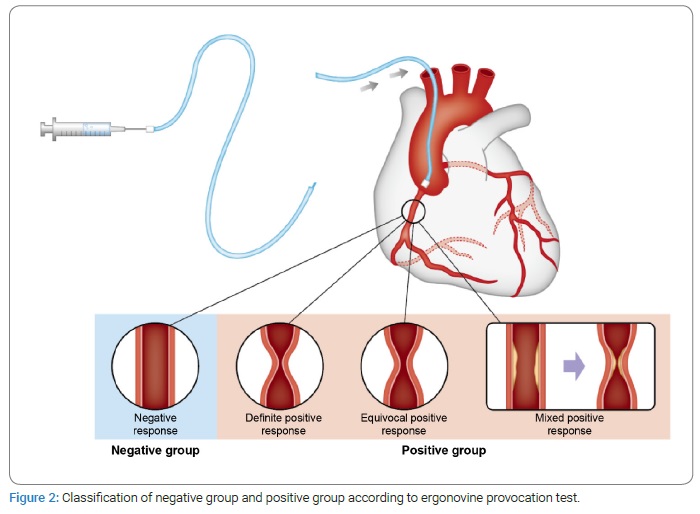
Classification of negative and positive provocation test results: A classic definition of a positive provocative test result for coronary artery spasm is that the test must induce all of the following responses to the provocative stimulus: (i) reproduction of the usual chest pain, (ii) ischemic ECG changes, and (iii) vasoconstriction of > 90% on angiography. The test result is considered equivocal if the provocative stimulus does not induce all three components. The consensus from the Coronary Vasomotion Disorders International Study Group (COVADIS) symposium was that vasoconstriction of > 90% is the angiographic threshold for the diagnosis of inducible spasm [21]. In this study, the provocation test results were classified into four groups as follows: negative, positive, equivocal positive, and mixed positive. Except for the mixed positive group, none of the groups had significant coronary artery stenosis on baseline CAG. The mixed positive group had 50%–70% coronary artery stenosis without stent implantation. The four groups are described as follows:
- The negative group: insignificant stenosis (stenosis of < 50%) with luminal narrowing of < 50% after an intracoronary ergonovine provocation test with or without atypical chest symptoms and signs.
- The definite positive group: insignificant stenosis (stenosis of < 50%) with luminal narrowing of > 90% after an intracoronary ergonovine provocation test with ischemic chest symptoms or signs.
- The equivocal positive group: insignificant stenosis (stenosis of < 50%) with luminal narrowing of 70%–90% after an intracoronary ergonovine provocation test with ischemic chest symptoms or signs.
- The mixed positive group: coronary artery stenosis (stenosis of 50%–70% without stent implantation) with luminal narrowing of > 90% after an intracoronary ergonovine provocation test with ischemic chest symptoms or signs. The positive provocation group included both the positive group and the equivocal positive and mixed positive groups. This classification was considered suitable for determining therapeutic approaches by a clinician (Figure 2).
Enzyme-linked immunosorbent assay (ELISA) for ALDH2: A double-sandwich ELISA was performed to determine human ALDH2 concentrations. All the experiments were performed using commercially available ALDH2 kits (Cusabio, Hubei, China), following the manufacturer’s protocols. Standards and samples were added to the precoated well and incubated for 2 hours at 37°C, then removed from the well. Biotin antibody was added to each well and incubated for 1 hour at 37°C. The well was washed three times with a wash buffer. A horse radish peroxidase-avidin conjugate was added to each well and incubated for 1 hour at 37°C. The well was washed five times with a wash buffer. A tetramethylbenzidine substrate solution was added to each well and incubated for 15 minutes at 37°C in the dark. A stop solution was then added to each well. Optical density was measured at a wavelength of 450 nm using a filter-based microplate photometer (MultiskanTM FC, Thermo ScientificTM, Massachusetts, USA).
Data analysis: R language version 3.1.1 (R Foundation for Statistical Computing, Vienna, Austria) was used for all statistical analyses. The differences between pairs of groups, analyzed and divided according to baseline characteristics and provocation results, were determined. The data obtained are expressed as mean ± SD or median for continuous variables and number (percentage) for categorical variables. One-way analysis of variance with a T-test was performed on the positive provocation group, which comprised the definite positive, the equivocal positive, and the mixed positive groups. Chi-square or Fisher exact test was performed to test the hypothesis of the association between categorical variables and vasospastic angina, defined according to the provocation test results. A p-value of < 0.05 was considered statistically significant.
Results
Demographic attributes and clinical parameters of the subjects: As described earlier, the final analysis included 195 subjects (119 men and 76 women), excluding those in which the human ALDH2 level could not be measured. The positive group accounted for 39% of the study subjects (76/195), and the negative group accounted for 61% (119/195). There were no significant differences between the groups’ risk factors for coronary artery diseases and medical history (Table 1).
Analysis of human ALDH2 concentration using the intracoronary ergonovine provocation test: Blood sampling via a radial catheter was performed before and after the intracoronary ergonovine provocation test with CAG. In the negative group, the ALDH2 concentrations before and after CAG were 13.73 ± 36.18 and 9.24 ± 24.76 ng/mL, respectively (Table 1). In the positive group, the ALDH2 concentrations before and after were 19.79 ± 41.13 and 9.79 ± 21.38 ng/mL, respectively. Moreover, in the positive provocation group, the change in ALDH2 concentration was greater than in the negative provocation group (standard error of the mean: −10.002 ± 4.53, p = 0.03 vs. −4.486 ± 3.469, p = 0.199; Table 2).
Discussion
The human ALDH2 gene, one of 19 functional ALDH genes, is expressed in many tissues, especially in the liver, kidney, and muscle. It is also present in organs with abundant mitochondrial content, including the heart and brain [22]. Guo et al. reported that ALDH polymorphisms were associated with an increased risk of coronary artery disease, possibly because of interfering high-density lipoprotein cholesterol (HDL-C) and endothelial asymmetric dimethylarginine levels [10,23]. Xu et al. reported that ALDH polymorphisms could be considered a genetic risk marker for the acute coronary syndrome [24]. Recent studies have shown that ALDH2 polymorphisms that inactivate ADLH2 may contribute to cardiovascular diseases. However, the role of ALDH2 in the cardiovascular system has not yet been ascertained. Moreover, human ALDH2 plasma levels have not been elucidated.
This is the first study investigating the ALDH2 concentration in human vasospastic angina.
According to the intracoronary ergonovine provocation test, the study showed that vasospasm was induced in 39% of the subjects. The baseline human ALDH2 concentrations differed between the negative and positive provocation groups. The mean baseline ALDH2 value was higher in the positive provocation group than in the negative provocation group (mean ± SD, 19.79 ± 41.13 vs. 13.73 ± 36.18; Figure 3).
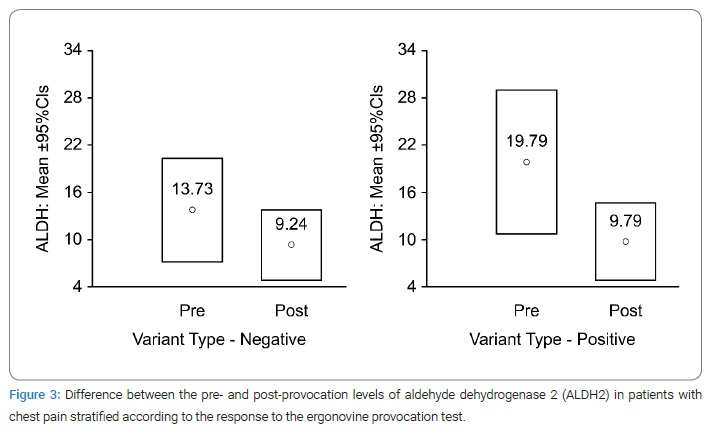
The difference in ALDH2 concentration was much larger in the positive provocation group than in the negative provocation group (−10.002 ± 4.53 vs. −4.486 ± 3.469; Table 2). This result could be due to the higher baseline ALDH2 concentration in the positive provocation group compared with that in the negative provocation group.
The human ALDH2 concentration in the peripheral blood after the provocation test was not significantly different because there is a large amount of mitochondrial ALDH2 in the liver and heart, and ALDH2 has rapid metabolic activity in these tissues.
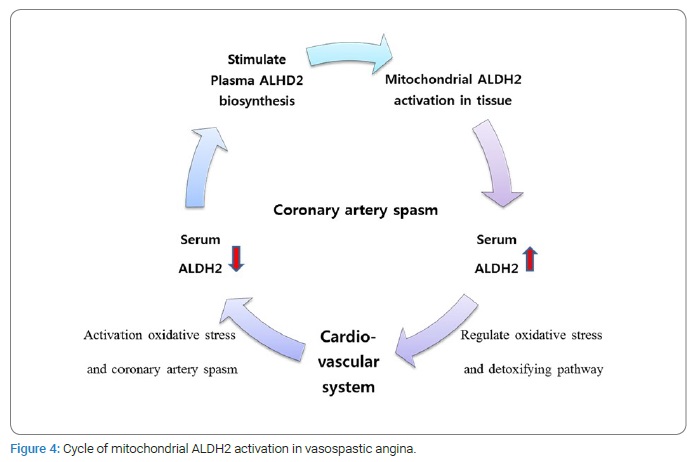
In summary, if a stimulus is applied to the cardiovascular system, serum ALDH2 is consumed to stimulate reactions. Thus, in a short time, the serum ALDH2 level is reduced to a lower level than is normal. Then, over time, a large amount of ALDH2 is activated and secreted into the circulating blood from mitochondrial tissue. When repeated, this cycle became a factor contributing to the elevated baseline ALDH2 level in the positive provocation group owing to coronary vasospasm (Figure 4).
Several limitations of the present study should be mentioned:
- The study was based on retrospective data.
- We did not perform the ELISA test repeatedly for accurate measurement of mitochondrial ALDH2 concentration in the plasma.
- Blood samples were stored for a long time, and the ELISA test could not detect deterioration of the plasma. Despite these limitations, this analysis could provide helpful information about the clinical features of patients who underwent the ergonovine provocation test and the investigation of human ALDH2 concentration.
Conclusions
ALDH2 concentration levels were more elevated in the positive provocation group than in the negative provocation group, and the difference in ALDH2 concentration was much larger in the positive provocation group.
Acknowledgments
The authors thank Dr. Jin Seo Yang, Department of Neurosurgery, Chuncheon Sacred Heart Hospital, College of Medicine, Hallym University for his assistance with illustrations.
Author contributions: Conceptualization: Kyu Tae Park, Hyun Hee Choi, and Kyung-Soon Hong; Data curation: Kyu Tae Park; Formal Analysis: Kyu Tae Park, Hyun Hee Choi, and Kyung-Soon Hong; Investigation: Kyu Tae Park, Hyun Hee Choi, and Kyung-Soon Hong; Methodology: Kyu Tae Park, Hyun Hee Choi; Project Administration: Kyu Tae Park, Hyun Hee Choi, and Kyung-Soon Hong; Writing – Original Draft: Kyu Tae Park and Kyung-Soon Hong; Writing – Review & Editing: Kyu Tae Park, Hyun Hee Choi and Seok Jun.
Consent: Written informed consent was obtained from the patient to publish this report in accordance with the journal’s patient consent policy.
Ethics statement: The study protocol was approved by the Cardiology Ethics Committee, institutional review board of Chuncheon Sacred Heart Hospital (IRB No. 2017-36). Informed consent was confirmed by the IRB.
Conflict of Interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Informed consent was obtained for this publication.
References
- Prinzmetal M, Kennamer R, Merliss R, Wada T, Bor N. Angina pectoris. I. A variant form of angina pectoris; preliminary report. Am J Med. 1959;27:375–388.
- Matta A, Bouisset F, Lhermusier T, Campelo-Parada F, Elbaz M, Carrié D, et al. Coronary artery spasm: new insights. J Interv Cardiol. 2020;2020:5894586.
- Picard F, Sayah N, Spagnoli V, Adjedj J, Varenne O. Vasospastic angina: A literature review of current evidence. Arch Cardiovasc Dis. 2019;112(1):44–55.
- Mizuno Y, Harada E, Kugimiya F, Shono M, Kusumegi I, Yoshimura M, et al. East Asians variant mitochondrial aldehyde dehydrogenase 2 genotype exacerbates nitrate tolerance in patients with coronary spastic angina. Circulation. 2020;84(3):479–486.
- Yasue H, Mizuno Y, Harada E. Coronary artery spasm - Clinical features, pathogenesis and treatment. Proc Jpn Acad Ser B Phys Biol Sci. 2019;95(2):53–66.
- Shin DI, Baek SH, Her SH, Han SH, Ahn Y, Park KH, et al. The 24-month prognosis of patients with positive or intermediate results in the intracoronary ergonovine provocation test. JACC Cardiovasc Interv. 2015;8(7):914–923.
- Boudou N, Despas F, Van Rothem J, Lairez O, Elbaz M, Vaccaro A, et al. Direct evidence of sympathetic hyperactivity in patients with vasospastic angina. Am J Cardiovasc Dis. 2017;7(3): 83–88.
- Hubert A, Seitz A, Pereyra VM, Bekeredjian R, Sechtem U, Ong P. Coronary artery spasm: the interplay between endothelial dysfunction and vascular smooth muscle cell hyperreactivity. Eur Cardiol. 2020;15:e12.
- Saito Y, Saito Y, Kato K, Kobayashi Y. Gender differences in factors associated with vasospastic angina. Int J Cardiol. 2022;349:7–11.
- Mizuno Y, Harada E, Morita S, Kinoshita K, Hayashida M, Shono M, et al. East Asian variant of aldehyde dehydrogenase 2 is associated with coronary spastic angina: possible roles of reactive aldehydes and implications of alcohol flushing syndrome. Circulation. 2015;131(19):1665–1673.
- Yasue H, Mizuno Y, Harada E. Association of East Asian variant aldehyde dehydrogenase 2 genotype (ALDH2* 2*) with coronary spasm and acute myocardial infarction. Adv Exp Med Biol. 2019;1193:121–134.
- Chang JS, Hsiao JR, Chen CH. ALDH2 polymorphism and alcohol-related cancers in Asians: a public health perspective. J Biomed Sci. 2017;24(1):19.
- Chen CH, Ferreira JCB, Joshi AU, Stevens MC, Li SJ, Hsu JHM, et al. Novel and prevalent non-East Asian ALDH2 variants; Implications for global susceptibility to aldehydes’ toxicity. E Bio Medicine. 2020;55:102753.
- Zhao T, Huang H, Tan P, Li Y, Xuan X, Li F, et al. Enhancement of solubility, purification, and inclusion body refolding of active human mitochondrial aldehyde dehydrogenase 2. ACS omega. 2021;6(18):12004–12013.
- Zhu Q, He G, Wang J, Wang Y, Chen W. Pretreatment with the ALDH2 agonist Alda-1 reduces intestinal injury induced by ischaemia and reperfusion in mice. Clin Sci (Lond). 2017;131(11):1123–1136.
- Zhang T, Zhao Q, Ye F, Huang CY, Chen WM, Huang WQ. Alda-1, an ALDH2 activator, protects against hepatic ischemia/reperfusion injury in rats via inhibition of oxidative stress. Free Radic Res. 2018;52(6):629–638.
- Papatheodorou I, Galatou E, Panagiotidis GD, Ravingerová T, Lazou A. Cardioprotective Effects of PPARβ/δ activation against ischemia/reperfusion injury in rat heart are associated with ALDH2 upregulation, amelioration of oxidative stress and preservation of mitochondrial energy production. Int J Mol Sci. 2021;22(12):6399.
- Chen CH, Ferreira JCB, Mochly-Rosen D. ALDH2 and cardiovascular disease. Adv Exp Med Biol. 2019;1193:53–67.
- Ushijima K, Ogami C, Tsuji Y, Nagano T, Fujimura A. Presence of the aldehyde dehydrogenase 2 variant ALDH2* 2 considerably increases EC50 of nitroglycerin. Eur J Clin Pharmacol. 2021;77(3):431–433.
- Kim M, Jang AY, Oh PC, Suh SY, Lee K, Kang WC, et al. Diagnostic and prognostic role of nitroglycerin-induced dilation in patients with suspected vasospastic angina, combined with ergonovine provocation test. Sci Rep. 2021;11(1):23834.
- Beltrame JF, Crea F, Kaski JC, Ogawa H, Ong P, Sechtem U, et al. International standardization of diagnostic criteria for vasospastic angina. Eur Heart J. 2017;38(33):2565–2568.
- Laskar AA, Younus H. Aldehyde toxicity and metabolism: the role of aldehyde dehydrogenases in detoxification, drug resistance and carcinogenesis. Drug Metab Rev. 2019;51(1):42–64.
- Zhang Y, Babcock SA, Hu N, Maris JR, Wang H, Ren J. Mitochondrial aldehyde dehydrogenase (ALDH2) protects against streptozotocin-induced diabetic cardiomyopathy: role of GSK3β and mitochondrial function. BMC Med. 2012;10:40.
- Xu F, Chen YG, Xue L, Li RJ, Zhang H, Bian Y, et al. Role of aldehyde dehydrogenase 2 Glu504lys polymorphism in acute coronary syndrome. J Cell Mol Med. 2011;15(9):1955–1962.
Keywords
Vasospastic angina; Aldehyde dehydrogenase 2; Vasospasm
Cite this article
Park KT, Seok J, Hong KS, Choi HH. The association between vasospasm and serum concentrations of aldehyde dehydrogenase 2 in patients with variant angina. Clin Case Rep J. 2022;3(6):1–8.
Copyright
© 2022 Hyun Hee Choi. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-4.0).






