Histiocytosis X: A Case Report of Langherans Cell Histiocytosis with the 3D Surgery
Umberto Autorino;
Fabio Passalacqua;
Caterina Iaquinta;
Davide Careglio;
Claudia Manini;
Alessandro Comandone;
* Claudio Caldarelli;
-
Umberto Autorino: Department of Maxillofacial Surgery, San Giovanni Bosco Hospital, ASL Città di Torino, Torino, Italy.
-
Fabio Passalacqua: Department of Maxillofacial Surgery, San Giovanni Bosco Hospital, ASL Città di Torino, Torino, Italy.
-
Caterina Iaquinta: Department of Maxillofacial Surgery, San Giovanni Bosco Hospital, ASL Città di Torino, Torino, Italy.
-
Davide Careglio: Department of Dentistry, Private Practice, Torino, Italy.
-
Claudia Manini: Department of Pathology, San Giovanni Bosco Hospital , ASL Città di Torino,Torino, Italy.
-
Alessandro Comandone: Department of Oncology, San Giovanni Bosco Hospital , ASL Città di Torino,Torino, Italy.
-
* Claudio Caldarelli: Department of Maxillofacial Surgery, San Giovanni Bosco Hospital, ASL Città di Torino, Torino, Italy.
Abstract
Background: Langerhans Cell Histiocytosis (LCH) is a relatively rare disorder with a strong inflammatory component. It has diverse clinical manifestations, which range from a single lesion or multiple bony lesions to severe multi-system involvement. Approximately 10% to 20% of cases of LCH occur in the jaw, with the posterior mandible being the most frequently involved site. The majority of patients are children younger than three years, and the incidence in adults is approximately 1-2/million. There is no universally accepted treatment protocol; treatment options include observation only, surgical curettage, radiation therapy, steroid injections, and chemotherapy.
Objective: Use the Computer-Aided Design and Computer-Aided Manufacturing (CAD/CAM) technology for the treatment of Langerhans cell histiocytosis in the adult mandible.
Case Presentation: We report on the case of a 56-year-old woman who presented an osteolytic lesion in the mandible body that was discovered incidentally during routine radiographic screening. Single-stage treatment was achieved by 3D surgery for bone resection, nerve preservation, and mandibular reconstruction by an autologous bone graft from the iliac crest.
Histological examination of the specimen confirmed the diagnosis of LCH. In addition, the patient did not show any recurrence in clinical and medical records at 12 months and 24 months after surgery.
Conclusion: 3D surgery is a potential treatment choice for this type of oral pathology as it allows for a tailored, safe, reliable therapy.
Introduction
Langerhans cells (LCs) are inflammatory dendritic cells that are derived from the bone marrow, migrate through the bloodstream to the epidermis of the skin and the epithelium of oral mucosa, and play an important role in the development of local immune response [1]. Clinically, its presentation can range from affecting only a single system, such as the skin or bone, to a life-threatening multi-system disease that involves other organs, including the lungs, spleen, bone marrow, and liver [2,3]. The incidence of LCH in adults ranges from 1 to 2 cases per million [2,4,5].
There is much debate about whether LCH represents a reactive or neoplasm process.
The term ‘histiocytosis’ refers to the proliferation of histiocytes and other inflammatory cells, whereas the letter ‘X’ was added to denote the unknown etiology of the disease [6,7].
Histologically, LCH, in general, is characterized by immature LCs proliferation accompanied by an infiltrate of lympho-monocytes together with eosinophilic and neutrophilic cells, which results in the destruction of the affected tissues [2]. As the atypical cellular proliferation of LCH occurs in various organs and tissues, clinical manifestations may be particularly different and complex [3]. The LCH study group divides LCH into single-system LCH and multi-system LCH; the single system is further subdivided into a single site and multiple sites, while the multi-system into low risk and high risk according to the involvement of some organs (liver, lungs, spleen, hematopoietic system), with high-risk patients presenting a higher mortality rate [3].
Skull manifestations commonly involve the calvaria, the temporal bone, and the jaws [8]. The jaws are involved in approximately 10% of cases; the mandibular body and angle are generally reported as more common sites than the maxilla, especially in patients over 20 years of age [9,10]. The lesion may be solitary or multiple. Multiple sites in both the mandible and the maxilla are usually involved; about 20% of patients have a polyostotic disease. In a series reported by Di Nardo and Wetmore, 86% of the mandibular lesions were unifocal [11].
There is not a gold standard treatment for LCH in the oral maxillofacial region; nevertheless, surgical therapy is included in the guidelines as the first choice treatment for the LCH single-system single site for oral localization [12]. The surgical therapy is varied and depends on the site and number of localizations; it can range from curettage up to resection followed by reconstruction [13]. Unfortunately, curettage can cause relapse, and the resection can be too aggressive and/or too wide, making the reconstruction challenging [14].
CAD/CAM surgery was progressively applied to maxillo-facial surgery over the years. As a result, different studies demonstrate the accuracy and the advantages of CAD/CAM-assisted surgery in oral and facial reconstruction [15–17]. The CAD/CAM technique allows the creation of Virtual Surgical Planning (VSP) and simulation by realizing and producing cutting guides and stereo-lithographic models. All of these tools enable the surgeon to achieve the best optimization for the treatment because they allow analyzing the patient-specific anatomy preoperatively in order better to understand the resection and better project the reconstruction; pre-bending of standard osteosynthesis plates, and production of customized implants, when achievable, reduce operative time and improve precision. As a result of a more precise resection and reconstruction in a shorter operative time, patient outcomes and quality of life are improved.
There are many studies showing the benefit of CAD/CAM use for treating oral cancer or facial deformities, but, at this time, there are no studies about the use of CAD/CAM for LCH treatment reported. The authors report a single system-single site LCH involving the mandible in a 56-year-old woman, with a special emphasis on surgical treatment by CAD/CAM surgery for the mandible resection and reconstruction.
Case Presentation
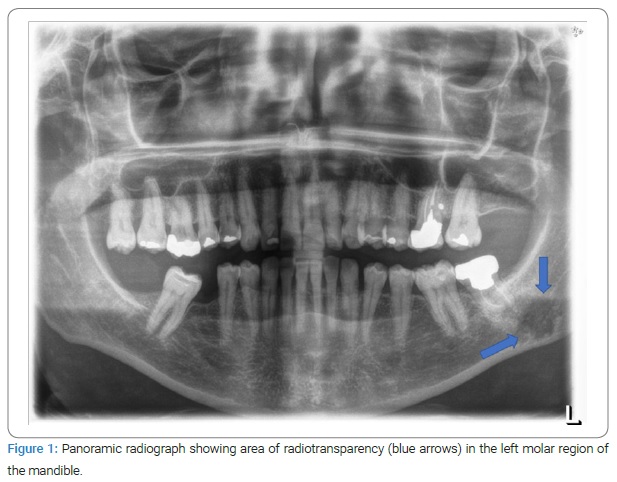
A 56-year-old female patient was referred with an extensive osteolytic lesion of the mandible. The patient was asymptomatic, and the lesion was found incidentally through a panoramic radiograph obtained for dental treatment purposes. The panoramic radiograph showed a large area of radio transparency in the left molar region of the mandible (Figure 1). A biopsy under local anesthetic was performed, and histological examination reported a pattern compatible with the diagnosis of LCH. Nuclear bone scanning with Technetium 99 m was then performed to investigate whether other bones or organs were involved, and no other localization was found.
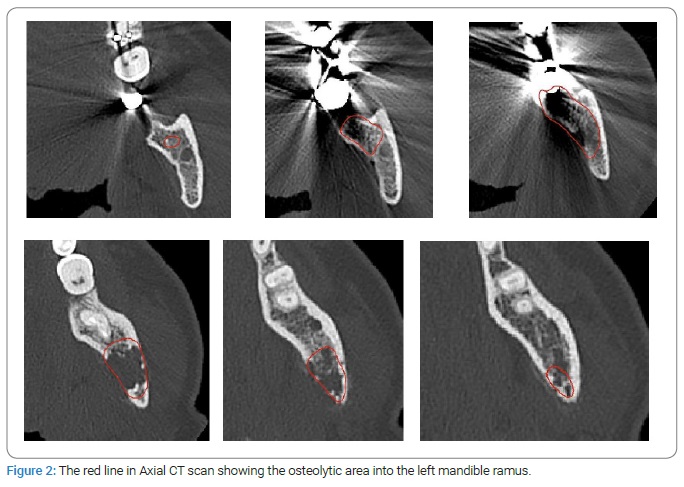
Based on the radiographic findings and clinical evidence, surgical treatment was planned by virtual surgical planning (VSP) and 3D printed surgical templates. To process VSP, pre-operative Computed Tomographic (CT) scans of head and neck and hip bone regions were performed with 0.6 mm slice thickness, and the digital imaging and communications in medicine (DICOM) data were imported (Figure 2). The VSP was simulated in HA3Dtm Ortopedia software, and 3D printed templates were designed and fabricated by medics tools for medicine.
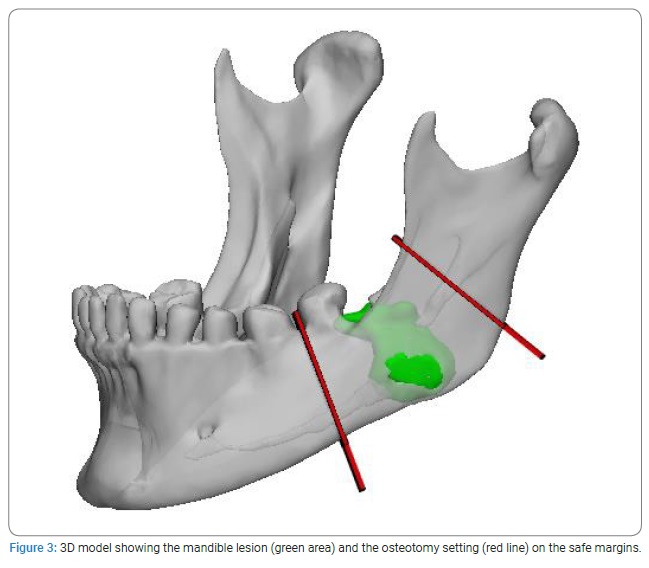
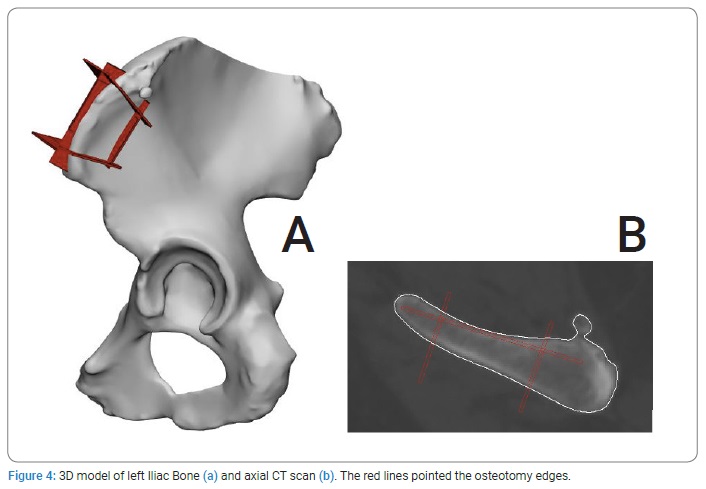
Virtual surgical planning: Frankfort Horizontal Plane (FHP), mid-facial plane (perpendicular to the FHP through the nasion), and coronal plane (perpendicular to the FHP through the Sella point) were used as reference planes to allow the study of the boundary and anatomic structure of the mandible lesion on the 3D model. After orientation, the planes for the osteotomy were settled, and the lesion resection was performed virtually (Figure 3). Then, the bone defect was defined. Next, the left iliac bone STL (Stereo Lithography interface format) file was successfully imported to plan the mandible reconstruction. Finally, the osteotomy planes for the iliac crest bone graft were set and cut (Figure 4). After the cutting step, the bone graft was virtually oriented to fit the defect (Figure 5).
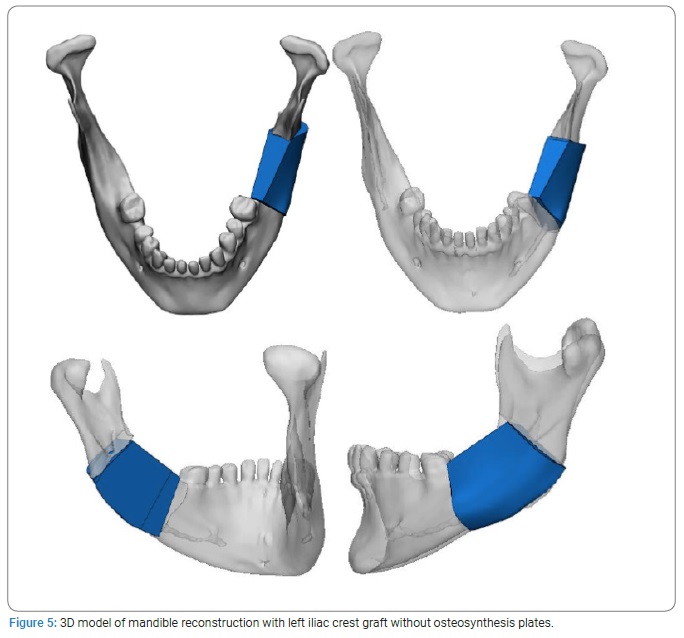
Guide design: To transfer the planning information to the operating room, the surgeon determined the cutting guides, who considered the clinical aspects of intraoperative feasibility. In addition, the cutting guide was packaged in a single piece in order to keep the two resulting mandibular stumps in a stable position. Finally, a 3D replica (stereolithographic model) of the reconstructed mandible with iliac graft positioned on site was fabricated in order to prebend one osteosynthesis mandibular plate that was sterilized preoperatively.
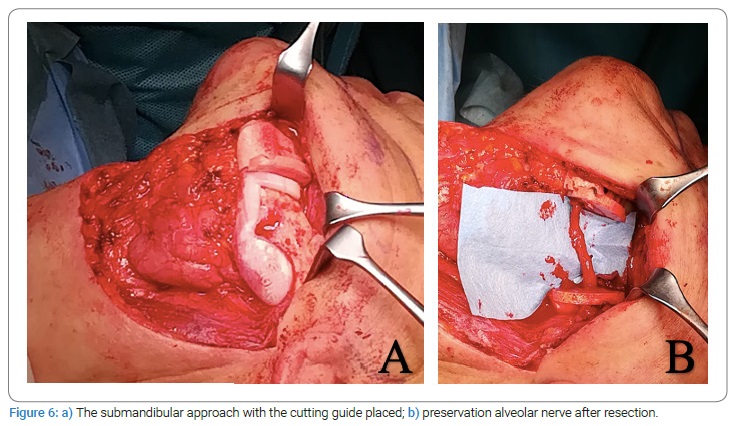
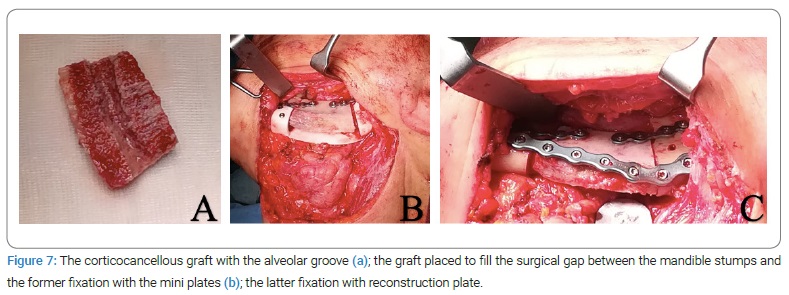
Surgical procedure: Under general anesthesia, the left mandible was accessed via a submandibular approach. Once the sub-mandibular access flap was raised, the periosteal flap was preserved, both on the lingual and the vestibular sides, in order to create a protective fold for the bone graft (Figure 6a, Figure 6b). Next, the diseased bone was removed by resecting the mandible segment according to the preplanned cutting and drilling guide (Figure 6a), and inferior alveolar nerve preservation was obtained by splitting the resection into two pieces (Figure 6b). Next, the corticocancellous graft for the reconstruction was harvested freehanded via the anterior approach to the iliac crest as the cutting guide could not be used because of its steric bulk. Next, the bone graft was shaped by drill on the lingual side to create a groove for the mandibular nerve (Figure 7a). Finally, it was planted to reconstruct the surgical gap with two mini plates onto the superior border with the cutting guide aid (Figure 7b), and the pre-bended reconstruction (mandibular) plate was placed on the inferior border (Figure 7c).
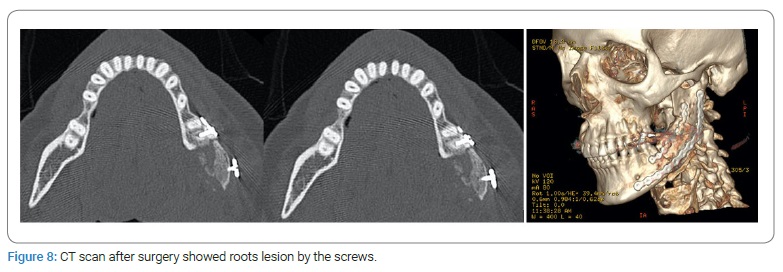
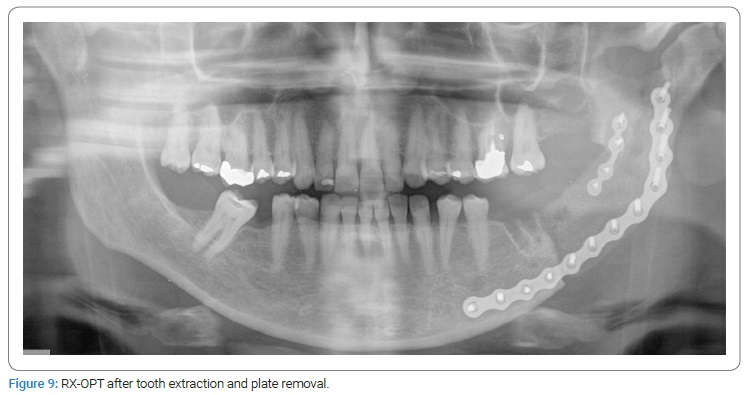
The patient’s postoperative course was complicated at one month by a left perimandibular abscess; a CT control scan showed an iatrogenic lesion of the first molar roots (Figure 8); the Authors performed tooth extraction and plate removal under local anesthesia followed by antibiotic therapy (Figure 9). After three weeks, the clinical abscess was resolved.
Temporary dysesthesia along the V3 branch of the trigeminal nerve was recorded but resolved spontaneously after three months.
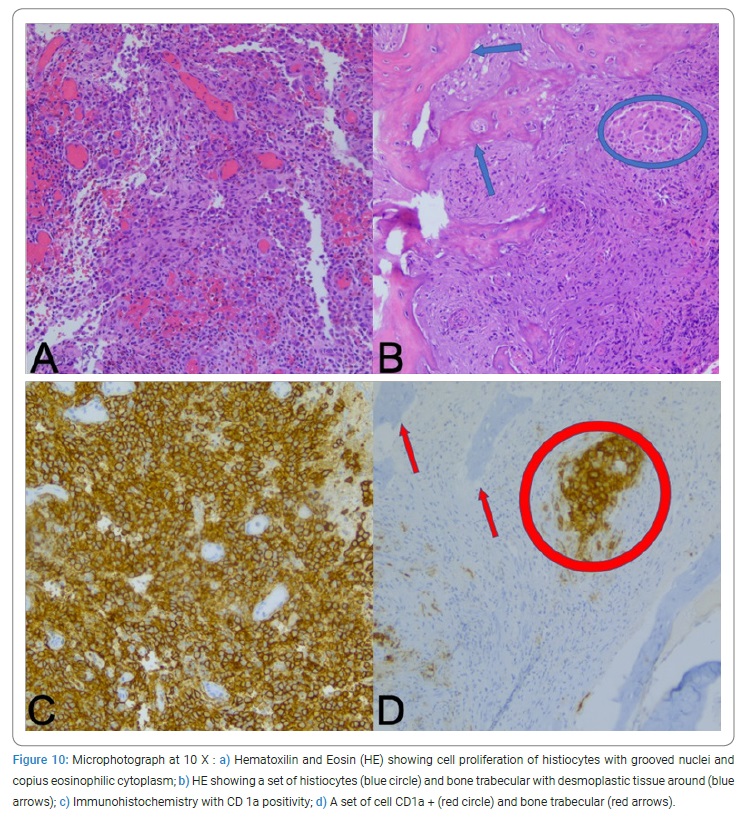
Histology revealed that the lesion was Langerhans Cell Histiocytosis (LCH) (Figure 10). Therefore, the patient was finally diagnosed with LCH single-system single-site (S-S).
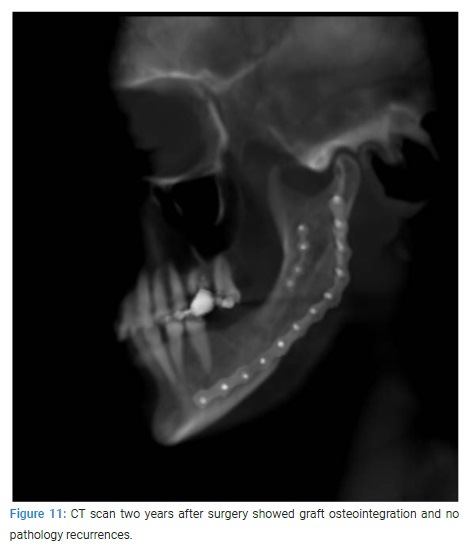
The patient did not show any recurrence in clinical and medical records at 12 months and 24 months after surgery (Figure 11). In addition, no deficit regarding the inferior alveolar nerve and no aesthetic disorders were recorded.
Discussion
Nowadays, there is a great debate about the gold standard therapy for patients with Langerhans Cell Histiocytosis (LCH) [2]. LCH treatment involves surgical interventions (from curettage to resection) [18,13], radiotherapy [3], and chemotherapy [3]; all of those can be used either alone or in a combined fashion.
The efficiency of radiotherapy of the jaws is discussed controversially: some studies have shown that radiotherapy might produce local control of bone lesions [3], while Watzke et al. have recorded completely unsuccessful results [19].
Surgical procedures are only called for if severe local problems occur, while radiation is reserved for patients with lesions that are either inaccessible or threatening vital structures.
Regarding surgical therapy, curettage is known to yield successful outcomes, but a number of recurrences have been reported [14]; on the other hand, bone resection requires demanding reconstructive procedures along with possible undesirable outcomes, such as paresthesia, infections, and excessive bone loss after reconstruction. The decision to undertake reconstructive surgery should take into consideration:
- Lack of evidence of systemic involvement of the disease.
- Lack of evidence of local or systemic recurrence.
- The risk of pathologic fracture of the mandible because residual bone thinness may support the need for reconstructive surgery.
Computer-assisted planning, surgery, and 3D printing for treating maxillo-facial pathologies have been documented extensively over the last decade [15–17]. However, at the time of publication, the authors are unaware of any paper reporting 3D-assisted surgical treatment for LCH of the mandible.
Our experience in the presented case shows that surgeons can clearly benefit from the technology described, but we hit some pitfalls. First of all, the unavailability of STL models of the plates did not make it possible to predict the steric size of the cutting guide with the placement of the plates, so we had to remove the guide to perform the osteosynthesis. Moreover, it was not possible to pre-bend the plates on virtual planning but on 3D replica only; consequently, it was not possible to match the holes of the plates with those of the cutting guide. So, after the bone resection, the cutting guide did not allow to perform osteosynthesis but checked the fitting of the bone graft only.
Another pitfall was the steric bulk of the cutting guide produced for the graft harvesting; it has to be noted that in some cases, surgeons will have to perform larger approaches due to the bulk of the guides, while we did not extend our approach and preferred free hand harvesting to avoid higher morbidity.
We could have prevented the pitfall using the STL of the plates for virtual planning to plan a cutting guide that provides housing for the plates and, consequently, a correct alinement of the holes. However, for the same reason, we could not predict the holes plates’ position, which led to damaging the first left inferior molar roots. Therefore, tooth extraction and plate removal under local anesthesia were necessary one month after the surgery. In this second surgical time, we checked the graft’s stability and the bone callus formation. The latter pitfall was due to lack of experience because preoperative planning takes time and requires a certain degree of mastery in computer sciences if done by the surgical team.
In conclusion, our case showed that surgical therapy (resection and reconstruction) offers a good choice to treat LCH single site-single system of the jaws. Computer-Aided Design and Computer-Aided Manufacturing implementation showed how technologies improve surgeon skills and patient outcomes but are not a tool for the unskilled surgeon in virtual surgical planning as issues during the surgical procedure are to be expected.
Conflict of Interest
We would like to confirm that the submitted manuscript has been read and approved by all the authors and that there are no conflicts of interest to be disclosed.
References`
- Bugshan AS, Alsaati MA, Syed FA, Almulhim KS, Abdulhady AI. Incidental diagnosis on orthopantomography of langerhans cell histiocytosis with multifocal jaw involvement: a case report of single-system disease. Am J Case Rep. 2020;21:e928307-1–e928307-6.
- Kobayashi M, Tojo A. Langerhans cell histiocytosis in adults: Advances in pathophysiology and treatment. Cancer Sci. 2018;109(12):3707–3713.
- Lajolo C, Campisi G, Deli G, Littarru C, Guiglia R, Giuliani M. Langerhans’s cell histiocytosis in old subjects: two rare case reports and review of the literature. Gerodontology. 2012;29(2):e1207–e1214.
- Carstensen H, Ornvold K. [Langerhans-cell histiocytosis (histiocytosis X) in children]. Ugeskr Laeger. 1993;155(23):1779–1783.
- Shi S, Liu Y, Fu T, Li X, Zhao S. Multifocal Langerhans cell histiocytosis in an adult with a pathological fracture of the mandible and spontaneous malunion: A case report. Oncol Lett. 2014;8(3):1075–1079.
- Jindal MK, Sharma VK, Ahmed I, Agrawal A. Langerhans cell histiocytosis of maxilla and mandible in 6 years old child: a case report. Int J Clin Pediatr Dent. 2009;2(2):45–49.
- Favara BE, Feller AC, Pauli M, Jaffe ES, Weiss LM, Arico M, et al. Contemporary classification of histiocytic disorders. The who committee on histiocytic/reticulum cell proliferations. Reclassification working group of the histiocyte society. Med Pediatr Oncol. 1997;29(3):157–166.
- Walker PD, Rosai J, Dorfman RF. The osseous manifestations of sinus histiocytosis with massive lymphadenopathy. Am J Clin Pathol. 1981;75(2):131–139.
- Albers SW, Francis DA, Alpert B, Laskin DM. Case 3. 2. Eosinophilic granuloma of the mandible. J Oral Surg Am Dent Assoc 1965. 1973;31(11):841–843.
- Dagenais M, Pharoah MJ, Sikorski PA. The radiographic characteristics of histiocytosis X. A study of 29 cases that involve the jaws. Oral Surg Oral Med Oral Pathol. 1992;74(2):230–236.
- DiNardo LJ, Wetmore RF. Head and neck manifestations of histiocytosis-X in children. Laryngoscope. 1989;99(7 Pt 1):721–724.
- Goyal G, Tazi A, Go RS, Rech KL, Picarsic JL, Vassallo R, et al. International expert consensus recommendations for the diagnosis and treatment of Langerhans cell histiocytosis in adults. Blood. 2022;139(17):2601–2621.
- Sbordone L, Guidetti F, Menchini Fabris GB, Sbordone C. Langerhans’ cell histiocytosis: a case report of an eosinophilic granuloma of the mandible treated with bone graft surgery and endosseous titanium implants. Int J Oral Maxillofac Implants. 2006;21(1):124–130.
- Terada T. Recurrent multifocal Langerhans cell histiocytosis of the mandible and maxilla in a 46-year-old man: a pathologic case report. Int J Clin Exp Pathol. 2013;6(5):939–942.
- Machado V, de Castro FBC, Jaeger C, Alfenas ER, Silva NRFA. CAD/CAM Beyond Intraoral Restorations: Maxillofacial Implant Guide. Compend Contin Educ Dent. 2019;40(7):466–472.
- Suojanen J, Hodzic Z, Palotie T, Stoor P. CAD/CAM engineered patient-specific impants as a reposition device in le fort i and modified subcondylar osteotomies: case report of facial deformity correction in acromegaly. Craniomaxillofacial Trauma Reconstr. 2020;13(3):226–236.
- Bettini G, Saia G, Valsecchi S, Bedogni G, Sandi A, Bedogni A. Three-dimensional CAD/CAM reconstruction of the iliac bone following DCIA composite flap harvest. Int J Oral Maxillofac Surg. 2021;50(1):32–37.
- Azariah EDS, Chandrasekaran D, Chinnaswami R, Balasubramaniam S, Jagdish E. “Histiocytosis X” - A Rare Case Report. J Clin Diagn Res JCDR. 2016;10(10):ZD19–ZD22.
- Watzke IM, Millesi W, Kermer C, Gisslinger H. Multifocal eosinophilic granuloma of the jaw: long-term follow-up of a novel intraosseous corticoid treatment for recalcitrant lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90(3):317–322.
Keywords
Histiocytosis X; 3D surgery; Mandible reconstruction; CAD/CAM surgery; Bone graft
Cite this article
Autorino U, Passalacqua F, Iaquinta C, Careglio D, Manini C, Comandone A, et al. Histiocytosis X: A case report of Langherans Cell Histiocytosis with the 3D surgery. Clin Case Rep J. 2022;3(6):1–8.
Copyright
© 2022 Claudio Caldarelli. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-4.0).











