Splenic Infarct following COVID-19 mRNA Vaccine: Is it Crohn’s or Vaccine-Induced Immune Thrombotic Thrombocytopenia?
* Aikaterini Mantaka;
Konstantina Bampili;
Dimitrios Papadomanolakis;
Areti Skordylaki;
-
* Aikaterini Mantaka: Department of Gastroenterology, “Saint George” General Hospital of Chania, Crete, Greece.
-
Konstantina Bampili: First Department of Internal Medicine, “Saint George” General Hospital of Chania, Crete, Greece.
-
Dimitrios Papadomanolakis: Department of Radiology, “Saint George” General Hospital of Chania, Crete, Greece.
-
Areti Skordylaki: Department of Blood Transfusion, “Saint George” General Hospital of Chania, Crete, Greece.
Abstract
Reports of thrombosis post-Coronavirus disease 2019 (COVID-19) vaccine have raised safety concerns. We report a rare case of possible vaccine-induced immune thrombotic thrombocytopenia diagnosed after the Pfizer vaccine in a Crohn’s disease patient who had been advised to discontinue his combination treatment (adalimumab plus azathioprine) before COVID-19 vaccination to improve antibodies response. Evidence-based medical advice by physicians remains crucial in vaccination campaigns.
Introduction
Reports of thrombotic events post Coronavirus disease 2019 (COVID-19) vaccine administration, especially for adults under 65 years, have caused safety concerns [1]. In addition, fear of severe side effects such as anaphylaxis, transverse myelitis, thromboembolic events, immune thrombocytopenia, pericarditis, myocarditis, Guillain-Barré Syndrome, etc., with fatal complications in some cases and misinformation of the general population, led to mistrust and consequent vaccine hesitancy [2,3].
Gaps in scientific knowledge and a lack of robust data for managing Inflammatory Bowel Disease (IBD) patients on biological medicines and immunomodulators at the beginning of the pandemic resulted in unjustified treatment discontinuations or alterations [4]. Fortunately, soon, IBD patients were reassured of the safety and efficacy of COVID-19 vaccines [5] irrespective of treatment type or active disease state, although the latter has been associated with an up to 3.6 times increased relevant risk of thrombosis [6].
We report a rare case of a patient with Crohn’s Disease (CD) and a possible vaccine-induced immune thrombotic thrombocytopenia (VITT) syndrome one week after the first dose of Pfizer COVID-19 vaccine.
Case Presentation
A 17-year-old male young patient with A1L3B1 CD presented to the emergency department with a fever up to 38.3oc, malaise, myalgia, and soft stools with mucus for the last three days. His symptoms had started one week after his first dose of Pfizer–BioNTech mRNA vaccine (BNT162b2). According to his recent medical history, he was on clinical and laboratory remission for the last three years with azathioprine 100 mg per day and adalimumab 40 mg every other week. However, he had been advised by his gastroenterologist to discontinue adalimumab one month before his vaccination and azathioprine on the day of his first dose with BNT162b2.
On physical examination, the patient had no abnormal findings apart from painless red eyes. His vital signs were BP: 105/58 mmHg, SatO2:95%, HR: 103/min, temperature: 38oc.
Initial laboratory tests revealed lymphopenia [WBC: 7310 K/μl; Neutrophils: 85.7% (6260); Lymphocytes: 8.2% (600)] and thrombocytopenia (PLT 148 K/μl), elevated D-dimers: 2640 FEU; CRP: 17 mg/dL (< 0.5) and ESR: 51 mm. Renal, liver function, and troponin-I levels were within normal limits. PCR test for SARS-CoV-2 was negative.
Stool cultures were negative for viral or microbial pathogens and parasites. Clostridium difficile stool culture, toxins A and B, or glutamate dehydrogenase were all negative. A chest X-ray and an abdominal ultrasound performed at the emergency room were normal. Ophthalmological examination on slit lamp was negative for any pathology.
The patient was administered ceftriaxone, doxycycline, metronidazole, and antithrombotic prophylaxis with fondaparinux 2.5 mg. After four days, the patient was still febrile with 3–4 soft stools per day and mild abdominal pain. His CRP was even more elevated up to 21.4 mg/dl, elevation of liver enzymes was also noticed (ALT up to 108 U/L, AST up to 137 U/L), and there was still thrombocytopenia with PLT range 145 K/μL-154 K/μL. Sequential blood cultures were negative for any pathogen, hepatotropic viral panel, Wright test, Coombs agglutination test, Mantoux test, and antibodies for zoonotic pathogens were all negatives (Table). Low titer IgM/IgG antibodies for Bartonella Henselae were not reproduced in consequent serologic reexamination. A second PCR COVID-19 test on day four after admission was again negative.
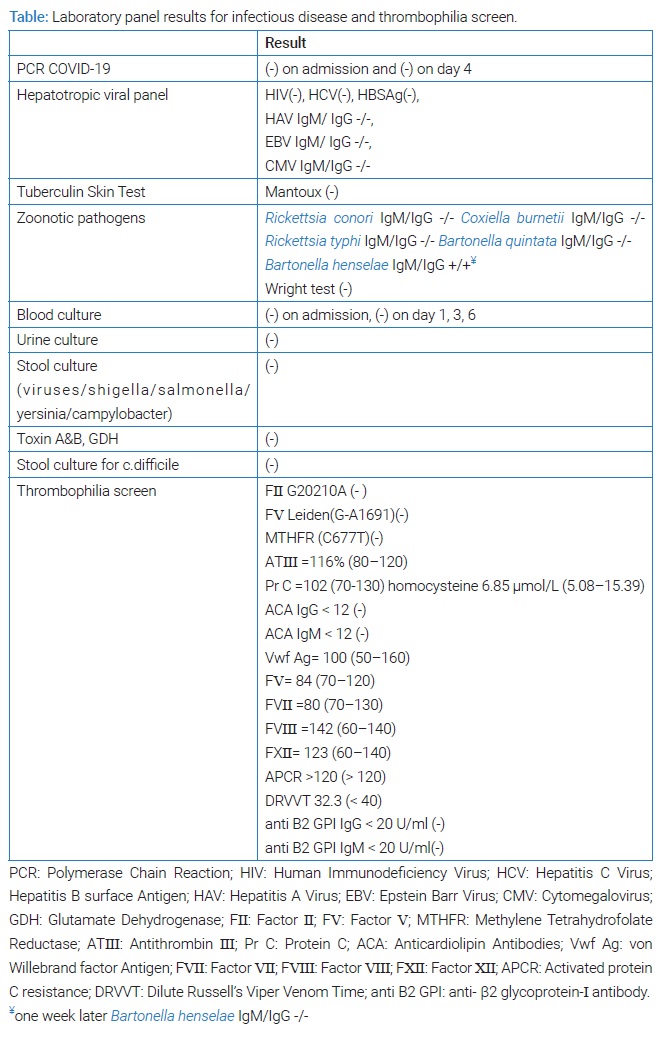
Sigmoidoscopy revealed mild erythema, decreased vascular pattern, and erosions in the descending and sigmoid colon and rectum, excluding severe exacerbation in the differential diagnosis of our patient’s fever and inflammatory syndrome. An abdominal CT scan with intravenous contrast medium was done and unexpectedly revealed a lack of attenuation in a small branch of the splenic vein and a splenic infarct in the upper pole of the spleen (Figure). Antithrombotic treatment was advanced to a therapeutic dose of fondaparinux 7.5 mg. Two days later, he was afebrile, and his inflammatory markers gradually decreased. Thrombophilia screen test revealed no evidence of inherited defects of anticoagulant or procoagulant factors (Table).
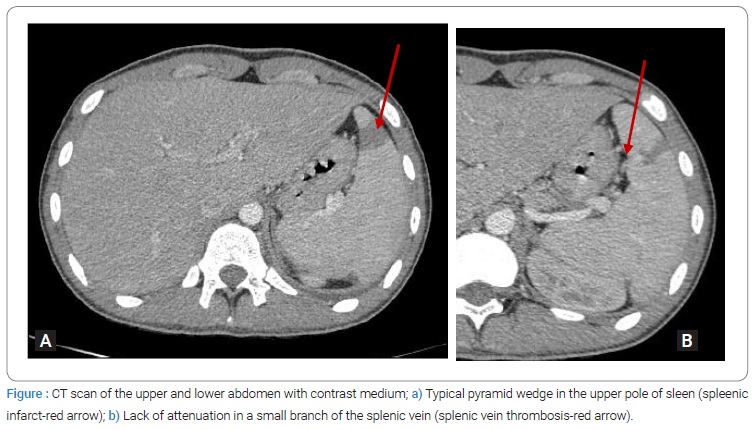
The patient was discharged after 10-day hospitalization with thrombocytosis PLT 548 K/μl (no Howell-Jolly bodies on peripheral blood smear), slightly elevated CRP 0.9 mg/dl, normal coagulation, and liver function tests. After 15 days, he commenced on adalimumab 40 mg/2w, and soon he was in clinical remission. He continued antithrombotic treatment for six months. However, a new CT scan with intravenous contrast after six months confirmed complete reperfusion of the spleen’s ischemic region; consequently, the hematologist advised him to stop anticoagulation. Being again on adalimumab 40 mg every two weeks, he is still in clinical and laboratory remission.
Discussion
One year after the declaration of COVID-19 as a pandemic, in March 2021, cases of a newly described syndrome of vein thrombosis in unusual sites, thrombocytopenia, and anti-platelet factor-4 antibodies (anti-PF4) were reported 4 days–28 days after the first dose of the AstraZeneca vaccine [1]. This complication after using adenovirus-based vaccines for SARS-CoV-2 (Astra Zeneca or Johnson & Johnson) was called VITT [1]. The mechanism of VITT seems to be similar to heparin-induced thrombocytopenia (HIT), with anti-PF4 in serum detected by Enzyme-Linked Immunosorbent Assays (ELISA) in the absence of heparin exposure [1].
Up to 22 July 2022, 32 Yellow Card reports of VITT following BNT162b2 have been reported in the United Kingdom [2]. However, to date, only five confirmed cases of VITT after mRNA COVID-19 (5 Moderna) vaccine have been reported to Vaccine Adverse Event Reporting System in the United States [7,8]. Therefore, based on collected data, an increased risk for VITT after mRNA COVID-19 vaccination has not been certified.
Based on diagnostic criteria for VITT, our case was a possible case of VITT, as we have excluded a severe CD flare or other thrombophilic conditions [1]. This young man met 4 out of 5 criteria, including a) timing (5 days–42 days after a COVID-19 vaccine), b) thrombosis (splenic vein), c) thrombocytopenia (platelets < 150 x 109/L), d) D-dimers 2000 FEU–4000 FEU, but e) unknown anti-PF4 antibodies. Anti-thrombotic treatment other than heparin was directly administered for a 6 months period, according to management guidelines.
The immunological interactions between IBD therapies and COVID-19 vaccines are not yet fully elucidated. However, accumulating evidence supports a lower pooled relative risk of seroconversion and attenuated serological responses to the SARS-CoV-2 vaccine in IBD patients treated with anti-tumor necrosis factor or in combination with immunomodulators [5]. The International Organization for the Study of Inflammatory Bowel Disease (IOIBD) reassured patients and healthcare professionals about the safety and efficacy of COVID-19 vaccines in IBD. Vaccinating all IBD patients as soon as the vaccine is available is recommended, regardless of IBD immune-modifying treatment type [5]. Nevertheless, the PREVENT study and a recent international web-based survey on the safety of COVID-19 vaccines had associated them with IBD relapse in 2.1% and less than 2% of participants, respectively [9,10].
Concerning the decision for this young man with CD in remission to discontinue anti-TNF and azathioprine one month before and on the day of COVID-19 vaccination, respectively, was not evidence-based, probably played a role in mild Crohn’s disease relapse, or could have implications in his thrombotic complications.
In conclusion, given the low prevalence of 0.73 per 100,000 cases of thrombotic thrombocytopenia after the Astra Zeneca COVID-19 vaccine [11], the even lower mRNA technology vaccines and the documented effective prophylaxis against severe forms of COVID-19 disease, vaccination remains a critical tool against SARS-CoV-2.
Conflict of Interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Informed consent was obtained for this publication.
References
- Cines DB, Bussel JB. SARS-CoV-2 vaccine-induced immune thrombotic thrombocytopenia. N Engl J Med. 2021;384(23):2254–2256.
- Research and analysis: Coronavirus vaccine - summary of Yellow Card reporting [Internet]. London: Medicines & Healthcare products Regulatory Agency; 2022.
- Dror AA, Eisenbach N, Taiber S, Morozov NG, Mizrachi M, Zigron A, et al. Vaccine hesitancy: the next challenge in the fight against COVID-19. Eur J Epidemiol. 2020;35(8):775–779.
- Konturek PC, Harsch IA, Neurath MF, Zopf Y. COVID-19 - more than respiratory disease: a gastroenterologist’s perspective. J Physiol Pharmacol. 2020;71(2).
- Siegel CA, Melmed GY, McGovern DP, Rai V, Krammer F, Rubin DT, et al; International Organization for the Study of Inflammatory Bowel Disease (IOIBD); International Organization for the Study of Inflammatory Bowel Diseases (IOIBD). SARS-CoV-2 vaccination for patients with inflammatory bowel diseases: recommendations from an international consensus meeting. Gut. 2021;70(4):635–640.
- Tan VP, Chung A, Yan BP, Gibson PR. Venous and arterial disease in inflammatory bowel disease. J Gastroenterol Hepatol. 2013;28(7):1095–1113.
- See I, Lale A, Marquez P, Streiff MB, Wheeler AP, Tepper NK, et al. Case Series of Thrombosis with Thrombocytopenia Syndrome after COVID-19 vaccination—United States, December 2020 to August 2021. Ann Intern Med. 2022;175(4):513–522.
- Su PH, Yu YC, Chen WH, Lin HC, Chen YT, Cheng MH, et al. Case Report: Vaccine-Induced Immune Thrombotic Thrombocytopenia in a Pancreatic Cancer Patient After Vaccination With Messenger RNA-1273. Front Med (Lausanne). 2021;8:772424.
- Weaver KN, Zhang X, Dai X, Watkins R, Adler J, Dubinsky MC, et al. Impact of SARS-CoV-2 Vaccination on inflammatory bowel disease activity and development of vaccine-related adverse events: results from prevent-COVID. Inflamm Bowel Dis. 2021;izab302.
- Ellul P, Revés J, Abreu B, Chaparro M, Gisbert JP, Allocca M, et al. Implementation and short-term adverse events of anti-SARS-CoV-2 vaccines in Inflammatory Bowel Disease patients: an international web-based survey. J Crohns Colitis. 2022;jjac010.
- Chan B, Odutayo A, Jüni P, Stall NM, Bobos P, Brown AD, et al. Risk of vaccine-induced thrombotic thrombocytopenia (VITT) following the AstraZeneca/COVISHIELD adenovirus vector COVID-19 vaccines. Science Briefs of the Ontario COVID-19 Science Advisory Table. 2021;2(28).
Keywords
Vaccine-induced immune thrombotic thrombocytopenia; Crohn’s disease; mRNA COVID-19 vaccine
Cite this article
Mantaka A, Bampili K, Papadomanolakis D, Skordylaki A, Anagnostopoulou E. Splenic Infarct following COVID-19 mRNA vaccine: Is it Crohn’s or vaccine-induced immune thrombotic thrombocytopenia? Clin Case Rep J. 2022;3(7):1–4.
Copyright
© 2022 Aikaterini Mantaka. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-4.0).


