Low-aggressive Long-term Continuous Hyperthermia in Combination with Extremely Low-Dose Chemotherapy for Prostate Cancer Patients at Medoc Health Clinic in Japan
* Takehiko Okamura;
Naruyama H;
Aoyama Y;
Maeda F;
Funabashi M;
Takekawa N;
Umemura Y;
Yoshida A;
-
* Takehiko Okamura: Department of Urology, Medoc Health Clinic, Japan; Department of Thermal Immunotherapy, Medoc Health Clinic, Japan.
-
Naruyama H: Department of Urology, Medoc Health Clinic, Japan; Department of Thermal Immunotherapy, Medoc Health Clinic, Japan; Department of Urology, Naruyama Hidamari Clinic, Japan.
-
Aoyama Y: Department of Thermal Immunotherapy, Medoc Health Clinic, Japan; Department of Surgery, Tokai Hospital, Japan.
-
Maeda F: Department of Thermal Immunotherapy, Medoc Health Clinic, Japan.
-
Funabashi M: Department of Thermal Immunotherapy, Medoc Health Clinic, Japan.
-
Takekawa N: Department of Thermal Immunotherapy, Medoc Health Clinic, Japan.
-
Umemura Y: Department of Thermal Immunotherapy, Medoc Health Clinic, Japan.
-
Yoshida A: Department of Thermal Immunotherapy, Medoc Health Clinic, Japan.
Abstract
According to the patient’s wishes, hyperthermia is an adjuvant therapy with an image as a treatment option; however, facilities that can provide this treatment are limited. We prioritize patients’ wishes at our hospital. Herein, we report the current situation along with reports of five patients who were treated with hyperthermia combined with extremely low-dose chemotherapy with in over 6 years. Five patients in their range of 42 years–72 years were histologically diagnosed with prostate cancer. The Gleason scores of the patients were 3 + 4, 4 + 4, 5 + 5, 8, and 5 + 5, respectively. No metastasis was observed in any case. The treatment interval was once a week immediately after the commencement of treatment, six cycles were administered as one course, and after 2–3 courses, the therapy was continued at a frequency of approximately once a month. Outpatient treatment was performed for up to 15 years, and the level of prostate-specific antigen was stable at approximately 1 ng/mL in four cases and 4 ng/mL–6 ng/mL in one case. Our treatment appears to be effective in patients with a high probability of developing Castration-Resistant Prostate Cancer as it suppresses disease progression over a long period.
Abbreviations
IPSA: Initial Prostate Specific Antigen; CAB: Combined Androgen Blockade Therapy; IMRT: Intensity Modulated Radiation Therapy; WW: Watchful Waiting; CDDP: Cis-Diammine Dichloro-Platinum (II); DXT: Docetaxel; GnRH: Gonadotropin Releasing Hormone; CRPC: Castration-Resistant Prostate Cancer; mEHT: Modulated Electro-Hyperthermia
Introduction
The goal of cancer treatment is to eradicate cancer through surgery, radiotherapy, or chemotherapy completely. The other goal is to live with cancer for a long period while suppressing cancer symptoms and controlling exacerbations, even without a complete cure.
Hyperthermia as a form of therapy has been used since the 1950s [1] and has long been used to treat various malignant tumors. Its effectiveness has been reported in combination with radiotherapy and anticancer drugs [2–4]. On the other hand, magnetic hyperthermia, which acts inside the cells directly, has also been tried previously and applied to various cancer types [5,6]. However, this treatment is an adjuvant therapy with an image as the treatment of choice at the patient’s request. Although the available facilities are gradually increasing, they are still limited. On the other hand, this treatment is one of the low-aggressive therapies with minimum side effects, does not decrease the effectiveness of the combination of low-dose anticancer drugs, and can be used for a long time. Furthermore, several patients request this treatment.
Insurance coverage for hyperthermia treatment was expanded in April 2020 in Japan, making it financially easier to receive treatment. Moreover, the need for hyperthermia treatment is gradually increasing owing to the rapid spread of information through the Internet. Therefore, in recent years, new models have been developed, and this therapy has entered a new generation.
In April 2021, we introduced a new model, “ASKF 8” (Shonai Creative Industry Co., Ltd. Japan) (Figure), in addition to the “Thermotron RF8” (Yamamoto VINITOR Co., Ltd. Japan), which we have been using for a long time, to make thermotherapy available to meet the needs of our patients. When localized, prostate cancer is considered a good indication for hyperthermia therapy. There have been reports in Japan since the 1980s [7] and several basic and clinical reports worldwide [8–11]. Herein, we report the cases of five patients with prostate cancer who were treated with hyperthermia in combination with extremely low-dose chemotherapy for more than 6 years. We also present the current status of treatment for cancer patients at our hospital.
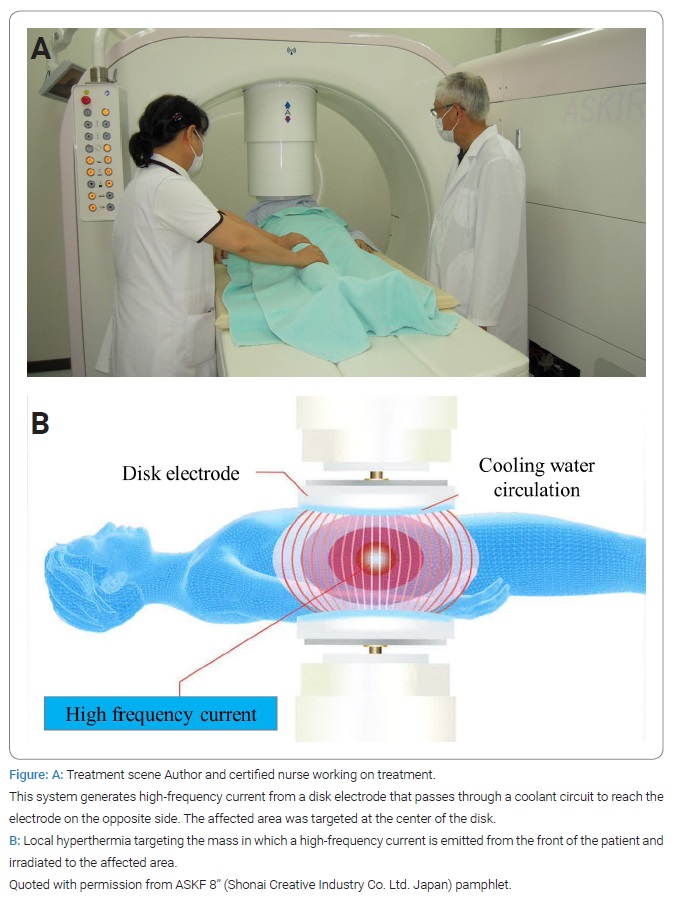
Case Presentation
We managed five histologically diagnosed prostate cancer cases in patients aged 42 years–72 years, and the age at the commencement of treatment was used. The Gleason scores of the patients were 3 + 4 = 7, 4 + 4 = 8, 5 + 5 = 10, 8 (no description given), and 5 + 5 = 10, respectively, and the initial Prostate Specific Antigen (iPSA) ranged from 4.9 ng/ml to 10.5ng/mL. None of the patients had metastases at diagnosis. Combined Androgen Blockade Therapy (CAB) was used as pretreatment in four patients, and Intensity-Modulated Radiation Therapy (IMRT) was administered to one patient.
The background for introducing hyperthermia was as follows: in one patient, watchful waiting was initially performed owing to the patient’s refusal of hormone therapy. The patient later accepted hyperthermia at the recommendation of his physician, who had been in charge of the patient since the initial diagnosis. The patient also underwent IMRT and CAB simultaneously. The other four patients were not convinced of the recommendation for drug therapy other than surgery or hormonal therapy and requested hyperthermia because they were concerned about the side effects of aggressive therapy. The second patient still received a combination of chlormadinone acetate, and his PSA levels were measured. The other three had not received hormonal therapy since the onset of hyperthermia therapy. All patients were treated with cis-di ammine dichloroplatinum (II) (CDDP), docetaxel (DTX), or both in alternating extremely low doses (CDDP, DXT 5 mg/body each), two patients were treated with IMRT, and one was treated with cyberknife (Table). Gonadotropin-Releasing Hormone (GnRH) was not administered to any of the patients.

In all but one case, the imaging diagnosis was based on CT and other imaging modalities that were regularly performed at our hospital. In addition, the third patient underwent periodic PSA and CT examinations during follow-up after IMRT at another hospital.
Results
After two or three courses, treatment was continued once a month, and the highest priority was given to the patient’s wishes. The duration of treatment was 9, 8, and 6 years in cases 1, 3, and 5, respectively, and two patients were treated as outpatients for > 15 years. During the treatment period, none of the four patients developed distant metastasis, their general condition was stable, and no side effects of anticancer drugs were observed.
Although accurate quality of life scores was not obtained, all five patients confirmed that their quality of life did not decline after treatment and that they were able to continue treatment for more than six years.
Discussion
At our institution, we administer low-aggressive cancer treatment in line with the patient’s wishes. Although this was a retrospective study and the number of patients was small, this report of five patients with prostate cancer confirmed that all patients had made satisfactory progress without developing Castration-Resistant Prostate Cancer (CRPC). Although hyperthermia alone is not sufficient to achieve the desired effect, many studies have reported its use in combination with other therapies.
In 2006, Baronzio et al. reported that hyperthermia improves the immune system of patients with cancer, in addition to the previously reported relationship between heat shock proteins and immunotherapy. They described it as a complex effect on cancer involving many immune response systems [12]. In 1995, Tanaka reported in detail the significance and role of hyperthermia in combination with radiation therapy [13], and in 2008, he reported its usefulness in the Hyperthermia Cancer Thermotherapy Guidebook [14]. In particular, there have been many reports on the usefulness of hyperthermia in combination with radiotherapy for the management of prostate cancer [9–11]. Regarding concomitant therapy with anticancer agents, Imada reported that concomitant therapy with low doses of anticancer agents in the same guidebook had fewer side effects and maintained its efficacy. However, he emphasized that localized disease is an important condition for useful effects [15].
In 2020, Oei et al. reviewed the mechanisms of hyperthermia, combined with clinical and experimental results [16]. They reported that the mechanisms of hyperthermia included inhibition of various DNA repair processes, direct and indirect reduction of hypoxic tumor cell percentages, enhancement of drug uptake, and increased perfusion and oxygen levels. As more research will be performed, a multidisciplinary approach with different modalities, including hyperthermia, might further increase antitumor effects and diminish normal tissue damage in the future.
This is a great advantage for outpatient treatment facilities, such as ours, that do not have inpatient wards, as it allows for less aggressive treatment with reduced patient burden and schedules according to the patient’s preference. In the same guidebook, Ueda states that prostate cancer is a good indication for patients with localized disease, good radiosensitivity, and concerns about the side effects of anticancer drugs and hormone therapy [17]. We have used hyperthermia therapy in combination with low-dose anticancer drugs for more than 6 years in patients with cancers other than prostate cancer, and compliance has been excellent. In particular, patients with prostate cancer are very sensitive to DTX, and the five cases that were administered DXT in this report were individual cases; therefore, we cannot make conclusions regarding the usefulness of DTX in these patients. However, none of the five patients has developed CRPC so far. Moreover, we believe that we can respond adequately to patients with CRPC who wish to receive less aggressive treatments.
In addition, Kanamori et al. reported a new concept of oncothermia (Modulated Electro-Hyperthermia, mEHT) in hyperthermia cancer therapy in detail [18]. Oncothermia, also known as mEHT, is a new treatment method for cancer hyperthermia. It is important to consider thermal effects and non-thermal effects (independent of temperature rise), such as inducing programmed cell death (apoptosis) of tumor cells and using low-power radio frequency to reduce side effects, such as burns. This therapy is a useful option for cancer treatment in combination with other therapies while reducing side effects as much as possible. Several recent studies on prostate cancer have also described the effectiveness of hyperthermia combined with other treatments [19–21]. However, in Germany, a 14-year study on prostate cancer focal treatment (23,677 cases) showed that hyperthermia accounts for a very small percentage (< 3%) and has not increased [22].
Many patients wish to avoid anticancer drug regimens and immuno-oncology drugs, which may cause unpredictable side effects. Given the ever-increasing number of patients who request so-called low-aggressive therapies, we are confident that their demand for hyperthermia therapy will increase in the future.
We sincerely hope that the number of facilities that provide hyperthermia therapy will gradually increase through the use of thermotherapy and that the environment in which patients can receive low-aggressive treatment will expand.
Conclusions
We have reported five cases of hyperthermia in combination with extremely low-dose chemotherapy for over six years. Our treatment appears to be effective in patients with a high probability of developing CRPCas it suppresses disease progression over a long period.
Conflict of Interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Informed consent was obtained for this publication.
References
- Gilchrist RK, Medal R, Shorey WD, Hanselman RC, Parrott JC, Taylor CB. Selective inductive heating of lymph nodes. Ann Surg. 1957;146(4):596–606.
- Schroeder C, Gani C, Lamprecht U, von Weyhern CH, Weinmann M, Bamberg M, et al. Pathological complete response and sphincter-sparing surgery after neoadjuvant radiochemotherapy with regional hyperthermia for locally advanced rectal cancer compared with radiochemotherapy alone. Int J Hyperth. 2012;28(8):707–714.
- Sreenivasa G, Hildebrandt B, Kummel S, Jungnickel K, Cho CH, Tilly W, et al. Radiochemotherapy combined with regional pelvic hyperthermia induces high response and resectability rates in patients with nonresectable cervical cancer > or = FIGO IIB “bulky”. Int J Radiat Oncol Biol Phys. 2006;66(4):1159–1167.
- Eckert F, Gani C, Kluba T, Mayer F, Kopp HG, Zips D, et al. Effect of concurrent chemotherapy and hyperthermia on outcome of preoperative radiotherapy of high-risk soft tissue sarcomas. Strahlenther Onkol. 2013;189(6):482–485.
- Sharma A, Cressman E, Attaluri A, Kraitchman DL, Ivkov R. Current challenges in image-guided magnetic hyperthermia therapy for liver cancer. Nanomaterials. 2022;12:2768.
- Włodarczyk A, Gorgon S, Radon A, Bajdak-Rusinek K. Magnetite nanoparticles in magnetic hyperthermia and cancer therapies: Challenges and perspectives. Nanomaterials. 2022;12:1807.
- Hirai M, Nakano M, Ushiyama T, Masuda H, Ohta N, Tajima A, et al. Trial of the hyperthermia treatment of prostatic cancer. Nihon Hinyokika Gakkai Zasshi. 1988;79(11):1761–1764.
- Pajonk F, van Ophoven A, McBride WH. Hyperthermia-induced proteasome inhibition and loss of androgen receptor expression in human prostate cancer cells. Cancer Res. 2005;65(11):4836–4843.
- Van Vulpen M, De Leeuw AAC, Raaymakers BW, Van Moorselaar RJA, Hofman P, Lagendijk JJW, et al. Radiotherapy and hyperthermia in the treatment of patients with locally advanced prostate cancer: Preliminary results. BJU Int. 2004;93(1):36–41.
- Müller AC, Zips D, Heinrich V, Lamprecht U, Voigt O, Burock S, et al. Regional hyperthermia and moderately dose-escalated salvage radiotherapy for recurrent prostate cancer. Protocol of a phase II trial. Radiat Oncol. 2015;10:138.
- Beck M, Ghadjar P, Mehrhof F, Zips D, Paulsen F, Wegener D, et al. Salvage-radiation therapy and regional hyperthermia for biochemically recurrent prostate cancer after radical prostatectomy (Results of the planned interim analysis). Cancers. 2021;13(5):1133.
- Baronzio G, Gramaglia A, Fiorentini G. Hyperthermia and immunity. A brief overview. In Vivo. 2006;20(6A):689–695.
- Tanaka Y. The meaning and the role of hyperthermia in multidisciplinary cancer therapy. Jpn J Hyperthermic Oncol. 1995;11(2):137–148.
- Tanaka R. Hyperthermia – Cancer hyperthermia guidebook. In: Japanese Society for Thermal Medicine. 1.03 Combination with Radiation. Japan; 2008. 8–11.
- Imada H. Hyperthermia – Cancer hyperthermia guidebook. In: Japanese Society for Thermal Medicine. 1.04 Combination with Anticancer Agents. Japan; 2008. 12–13.
- Oei AL, Kok HP, Oei SB, Horsman MR, Stalpers LJA, Franken NAP, et al. Molecular and biological rationale of hyperthermia as radio- and chemosensitizer. Adv Drug Deliv Rev. 2020:163–164:84–97.
- Ueda K. Hyperthermia – Cancer hyperthermia guidebook. In: Japanese Society for Thermal Medicine. 2.13 Urological Tumor. 2008:88–91.
- Kanamori M, Sato T, Shima T, Saitoh J, Andocs G, Kondo T. “Oncothermia” modulated electro-hyperthermia: current status and future prospects. Thermal Med. 2021;37:1–14.
- Nakahara S, Ohguri T, Kakinouchi S, Itamura H, Morisaki T, Tani S, et al. Intensity-modulated radiotherapy with regional hyperthermia for high-risk localized prostate carcinoma. Cancers. 2022;14(2):400.
- Le Guevelou JL, Chirila ME, Achard V, Guillemin PC, Lorton O, Uiterwijk JWE, et al. Combined hyperthermia and radiotherapy for prostate cancer: A systematic review. Int J Hyperthermia. 2022;39(1):547–556.
- Kawai N, Nagai T, Naiki-Ito A, Iida K, Etani T, Naiki T, et al. Combination therapy with radiation and hyperthermia-induced clinical complete response of small cell carcinoma of prostate. IJU Case Rep. 2022;5(2):113–116.
- Flegar L, Zacharis A, Aksoy C, Heers H, Derigs M, Eisenmenger N, et al. Alternative- and focal therapy trends for prostate cancer: a total population analysis of in-patient treatments in Germany from 2006 to 2019. World J Urol. 2022;40(7):1645–1652.
Keywords
Prostate cancer; Long-term continuous hyperthermia; Combination chemotherapy; Low aggressive therapy
Cite this article
Okamura T, Naruyama H, Aoyama Y, Maeda F, Funabashi M, Takekawa N, et al. Low-aggressive long-term continuous hyperthermia in combination with extremely low-dose chemotherapy for prostate cancer patients at medoc health clinic in Japan. Clin Case Rep J. 2022;3(7):1–5.
Copyright
© 2022 Takehiko Okamura. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-4.0).



