Abstract
Introduction: Kidney transplant recipients have a 2 to 5-fold risk of developing malignancy due to the long-term use of immunosuppressive agents for preserving graft function.
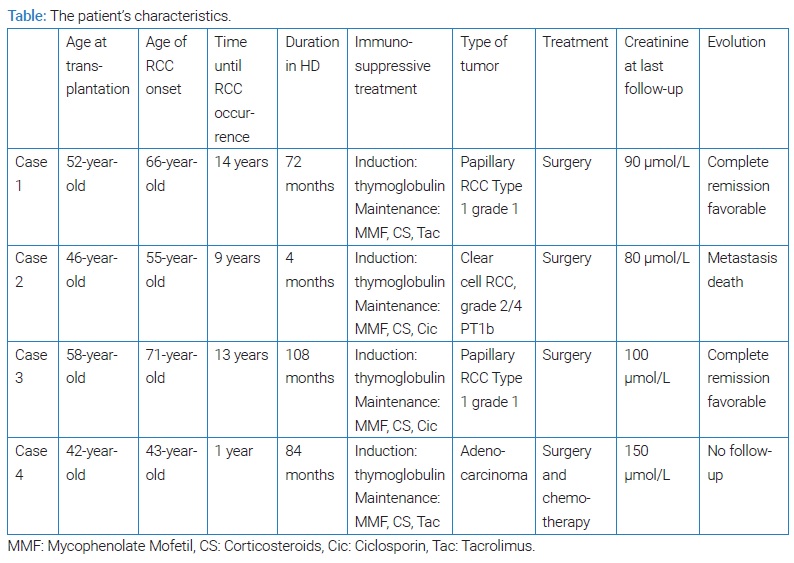
Objective: We report our experience with diagnosing and treating four cases of kidney cancer after a renal transplant.
Methods: Four patients (0, 83%) developed a tumor on the native or graft kidney. We retrospectively evaluated the clinical features and the outcome of these renal transplant recipients.
Results: The overall incidence of kidney cancer in our series was 0, 83%. Three patients developed cancer of the native kidney, and one patient had allograft cancer. The main histological type was papillary carcinoma. The tumors were discovered by Routine ultrasounds in all cases. Radical nephrectomy was performed on the native kidney in three cases. One patient underwent partial nephrectomy, then later on radical nephrectomy of the allograft due to recurrence and progression of cancer. One patient died after the main follow-up of 2 years due to hepatic metastasis.
Conclusion: Kidney cancer after kidney transplantation is associated with multiple complex risk factors. Radical nephrectomy is the treatment of choice with minimization or discontinuation of calcineurin inhibitors and the subsequent introduction of mTORi. However, routine and lifelong screening of both native and renal transplant allografts would allow earlier diagnosis and management of this malignancy.
Introduction
Kidney transplantation is the treatment of choice for patients with End Stage Renal Disease (ESRD). It improves survival and quality of life [1]. However, kidney transplant recipients have a 2 to 5-fold risk of developing malignancy due to the long-term use of immunosuppressive agents for preserving graft function [2]. Malignancy contributes to mortality in 9% to 16% of renal transplant recipients [3]. Urinary tract cancer is reported as the third most common malignancy after kidney transplantation [4]. Studies demonstrated that the incidence of Renal Cell Carcinoma (RCC) is 15 times higher in the native kidneys of Renal Transplant Recipients (RTR) than in the general population [5]. Here, we report our experience treating four kidney cancer cases after renal transplant and its management.
Methods
Between June 1998 and December 2020, 480 patients underwent kidney transplantation at our institution. Of these, four patients (0, 83%) developed a tumor on the native or graft kidney. We retrospectively evaluated the clinical features and the outcome of these renal transplant recipients. This study was approved by the local Ethics committee (Protection committee for Sud Persons, approval number CPP/SUD: 220/2021).
Statistical analysis was not performed in our study. However, informed consent was obtained from all our patients of their close family members.
Case Presentation 1
A 71-year-old male with ESRD since 1996 secondary to nephrosclerosis received a deceased donor renal transplant in November 2002. The immediate post-transplant period was marqued by acute obstructive renal failure due to a blood clot in the ureter with favorable outcomes after surgery. At follow-up, his baseline creatinine levels varied in the range of 100 µmol/L–140 µmol/L. A routine abdominal ultrasound in October 2016 demonstrated a heterogeneous mass in the left native kidney. A Computerized Tomography scan (CT scan) was performed, which revealed a 3 cm × 3.1 cm × 3.3 cm mass in the left native kidney. A nephrectomy of the left native kidney was performed, and a Histopathology Examination (HPE) confirmed papillary RCC Type 1 grade 1 with negative margins. The postoperative period was uneventful. He was switched to Rapamune instead of tacrolimus. Four-year later, in December 2020, the detection of cancer recurrence or extension was negative: CT scan of the abdominopelvic region was normal. In his last follow-up in May 2021, he was doing well, and his creatinine ranged from 90 µmol/L–110 µmol/L.
Case Presentation 2
A 57-year-old male suffering from ESRD secondary to familial nephropathy received a renal transplant from an unrelated living donor in June 2006. At follow-up, his baseline creatinine varied in the range of 80 µmol/L–110 µmol/L. In September 2015, during a routine abdominal ultrasound, he was incidentally diagnosed with a renal mass in the lower pole of the left native kidney. CT scan confirmed a solid enhancing lesion that measures 6.5 cm × 5 cm. In October 2015, a radical nephrectomy of the left native kidney was performed. The postoperative period was uneventful. The HPE confirmed a 7 cm clear cell RCC, grade 2/4 PT1b, with negative margins. He was switched to Rapamune instead of ciclosporin. His baseline creatinine post-transplant was maintained in the range of 80 µmol/L–100 µmol/L. He was lost to follow-up for two years and then died in 2018 due to hepatic metastasis.
Case Presentation 3
A 72-year-old male suffering from ESRD secondary to chronic interstitial nephropathy received a renal transplant from a related living donor in February 2006. The abdominal ultrasound before the renal transplant showed multiple cortical cysts in the native kidney, the largest of which is 2 cm. Post-transplant, his baseline creatinine varied in the range of 150 μmol/L–167 μmol/L. He was treated for four urinary tract infections. A retrograde cystography showed a grade four Vesicoureteral reflux on the graft and a significant post-voiding residue, and a diverticula bladder. Bladder augmentation and ureteral reimplantation were performed in 2016 with a favorable outcome.
In May 2019, during a routine abdominal ultrasound, he was incidentally diagnosed as having a renal mass measuring 7 cm. CT scan confirmed a solid enhancing lesion that measures 7 cm × 6.8 cm. In June 2019, a radical nephrectomy of the native kidney was performed. The postoperative period was uneventful. The HPE confirmed a 7 cm papillary RCC Type 1 grade 1 with negative margins. He was switched to Rapamune instead of ciclosporin. During his last follow-up in February 2021, he was doing well. His baseline creatinine post-transplant was maintained in the range of 100 µmol/L–120 µmol/L.
Case Presentation 4
A 49-year-old female suffering from ESRD secondary to undetermined nephropathy received a renal transplant from a deceased donor in February 2006. The donor was a 40-year-old male with a history of alcohol and tobacco consumption.
The postoperative period was uneventful, and her post-transplant baseline creatinine varied in the range of 100 μmol/L–150 μmol/L. In July 2008, a routine abdominal ultrasound was normal except for increased renal resistive index. A CT scan of the abdomen and pelvis was then performed to detect a Transplant Renal Artery Stenosis (TRAS), and it did not show any abnormalities. In December 2008 (13 months after the renal transplant), the patient was hospitalized for acute obstructive renal failure with creatinine levels = 1000 μmol/L treated by a percutaneous nephrostomy. An abdominal CT scan showed a small solid enhancing mass located at the pyelo ureteral junction of the renal graft. The treatment consisted of uretero-pyelotomy with ureteropyelic anastomosis. The postoperative period was uneventful, and the creatinine level was back at 150 μmol/L. BK virus status was checked and came back negative. The HPE confirmed a 2 cm moderately differentiated adenocarcinoma with negative margins. She was switched to Rapamune instead of tacrolimus. In Mars 2009, the patient underwent a radical nephrectomy of the renal graft due to recurrence and extension of the tumor (bone metastases). She also received carboplatin-based chemotherapy. At the last follow-up in our unit in 2014, she was doing well. There was no locoregional recurrence with stabilization of bone metastases. Since then, the patient has been lost to follow-up.
Discussion
RCC is the most common solid-organ malignancy after kidney transplantation [1,6]. Many studies showed that kidney transplant recipients have a relatively increased risk of 5 to 10-fold for RCC compared with an aged-matched general population [7–9]. In the current series, the incidence of de novo renal tumors is 0, 83%. The prevalence varies between 0.34% and 5.8%, and this disparity depends on whether screening is performed [10]. Chewcharat et al. [1] found in a meta-analysis of 22 articles, including 320.190 kidney transplant recipients, an incidence of 0.7% of RCC after renal transplantation.
In our series, the median time from transplantation to the onset of RCC was 111 months (12 months–168 months) which is a longer time than that reported in the literature. However, in other studies, the time between transplantation and the development of graft RCC is variable (9 months–228 months), similar to our study. A short period of time following the transplantation suggests that it is transmitted from the donor [11–13].
RCC usually occurs in native kidney transplants (0.7%), but it can also occur in the allograft (0.2%). In our case series, we only reported one case of adenocarcinoma of the allograft. RCC after transplantation is more frequent in younger male individuals as compared to the general population [13]. This can be explained by surveillance bias. In our study, three out of 4 patients were males, and two patients were under the age of 60-year-old at the onset of RCC.
The most frequent type of cancer is papillary RCC compared to the general population and dialysis patients. It accounts for more than 30% of cases suggesting a role of immunosuppression in the development of this type of cancer [14–16]. In our series, papillary de novo RCC on the native kidneys was the most frequent type of tumor, occurring in two out of four patients.
Most RCC in kidney recipients are incidental low–stage, low–grade tumors with a good prognosis [12]. These tumors are generally small and asymptomatic. Therefore, they are usually diagnosed upon routine imaging, as seen in our study.
Many risk factors have been reported in post-transplant RCC, such as older age, male gender, excess body weight, smoking, positive family history, arterial hypertension, previous RCC prior to kidney transplantation, and longer pretransplant dialysis duration. However, Acquired Cystic Kidney Disease (ACKD) is one of the main risk factors that has been reported [17].
Findings from the USRDS [18] suggest a higher incidence of RCC after deceased kidney transplantation than after living transplantation, highlighting the importance of dialysis duration as a relevant risk factor.
The association between malignancies and immunosuppression in kidney transplants is well established. Calcineurin inhibitors promote tumor growth, metastasis, and angiogenesis due to aberrant production of cytokines and overproduction of TGFβ [19,20].
In our study, our patients had many risk factors as described in studies. In fact, they were all on calcineurin inhibitors, and 3 out of 4 patients were males. In addition, the duration in HD was over five years in 3 cases, and the age of the onset of the tumor was over 60 in 3 cases.
Some studies suggest that patients with glomerular diseases are at higher risk of RCC [21,22]. In fact, the loss of podocytes that envelope glomerular capillaries can often lead to renal cysts [28]. In contrast, patients with ESRD secondary to diabetes or autosomal polycystic disease have a lower risk of RCC. In addition, repeat kidney transplantation and kidneys from older or male donors are also risk factors for RCC [9,23,24].
Thus, some risk factors are modifiable such as the duration of dialysis, which can be shortened by earlier planning of transplantation and encouraging living kidney donation and preemptive transplantation. In addition, immunosuppressive treatment can also be adjusted through level monitoring of immunosuppressive drugs.
The standard surgical treatment for native kidney carcinoma is radical nephrectomy [25]. Radical nephrectomy on the native kidney was performed for three patients in our study. In the series conducted by Tillou et al. [12], 48% of patients who developed allograft malignancies underwent a total graft nephrectomy, 44% nephron-sparing surgery, 6% radiofrequency ablation and the overall estimated malignancy-free survival was 0.4%. In our series, Nephron sparing surgery in patient 4 was not a good alternative to total graft nephrectomy in small tumors of the graft. We noted a recurrence and extension of the tumor shortly after, and the patient had to undergo a radical nephrectomy of her allograft. However, in many case series, this attitude showed good results with no tumor recurrence and allowed to preserve allograft to function at the same time [26].
The management of immunosuppression after diagnosis of RCC is not well established, and to our knowledge, there are no specific recommendations in the literature concerning the management of anti-rejection therapy. Mycophenolate mofetil does not seem to be associated with an increased risk of any malignancy [19,27]. Thus, in de novo low-stage low-grade RCC without metastasis, reduction of anti-rejection therapy with close follow-up is wise. In contrast, switching to a free calcineurin inhibitor immunosuppressive regimen in aggressive and metastatic forms of RCC is a reasonable option. Sirolimus and its derivatives are promising therapeutic agents with both immunosuppressant and anti-tumor properties. Many authors have demonstrated that the incidence of cancers was low in patients receiving mTOR inhibitors (mTORi) [28]. Therefore, the switch from a calcineurin inhibitor regimen to an mTOR regimen could be an interesting alternative option for patients with de novo renal malignancies after transplantation. This was the case for all our patients who were switched from a calcineurin inhibitor regimen to sirolimus.
Systematic kidney transplant recipients screening by annual abdominopelvic US or CT scan is recommended in France, but the American KDIGO clinical practice guidelines and the American Society of Transplantation guidelines do not recommend such screening [29].
However, considering the risk of mortality and morbidity, kidney transplant recipients should benefit from a regular ultrasound screening. Likewise, patients with a longer duration of pretransplant dialysis should benefit from more frequent ultrasound monitoring. At our center, an annual ultrasound screening of allograft and native kidneys is performed.
Malignant tumors are theoretically more aggressive in transplant patients compared to the general population and dialysis patients due to the immunosuppressed state. However, the potential aggressivity depends on the grade and size of the tumors.
Usually, the prognosis of low-stage RCC in the native kidneys of renal transplant recipients is favorable [21]. In contrast, metastatic RCC has a very poor prognosis [30]. However, in a large French multicenter study, they found better survival in transplant patients with RCC compared to a dialysis cohort. This can be explained by the smaller size of tumors and early diagnosis [14].
Cite this article
Abid H, Yaich S, Fendri B, Chaker H, Toumi S, Dammak N, et al. Kidney cancers in renal transplant recipients: a report of 4 cases and a review of the literature. Clin Case Rep J. 2023;4(1):1–5.