Abstract
Background: Infliximab is an anti-tumor necrosis factor α (anti-TNFα) monoclonal antibody for treating moderate to severe Crohn’s disease. Rarely, infliximab has been associated with Drug-Induced Aseptic Meningitis (DIAM), a potentially serious side effect.
Case presentation: A 43-year-old woman with a medical history of Crohn’s disease was treated with infliximab for ten months. She developed headaches, fever, and confusion 24 hours after the last infliximab infusion. Her neurological examination and the complete laboratory work-up were compatible with infliximab-induced aseptic meningitis. Infliximab was discontinued, and the symptoms resolved within ten days post-infusion.
Conclusion: Infliximab-associated meningitis is a rare disease that mimics infectious meningitis.
Introduction
Infliximab (IFX) is a chimeric monoclonal antibody against tumor necrosis factor α (anti-TNFα) used to treat moderate to severe Crohn’s disease (CD) [1]. Although it is generally well tolerated in most patients, IFX has been associated with Drug-Induced Aseptic Meningitis (DIAM), a rare but serious adverse event. There have been only nine previously reported cases [2–9].
Case Presentation
A 43-year-old woman presented with fever, headache, and confusion during treatment for a CD with IFX. Symptoms developed 24 hours post-infusion. The patient experienced muscle weakness, nausea, and vomiting two days after each of the previous IFX infusions (5 mg/kg every eight weeks). Monotherapy with IFX was initiated ten months before, and the patient was in remission. The brain’s neurological examination and Magnetic Resonance Imaging (MRI) was normal.
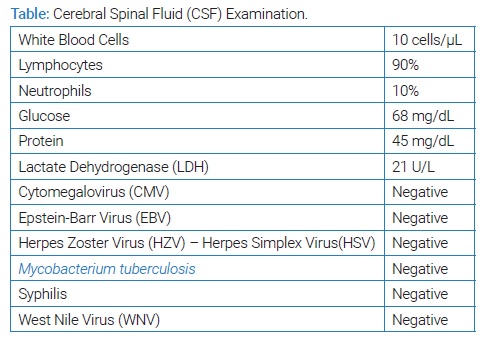
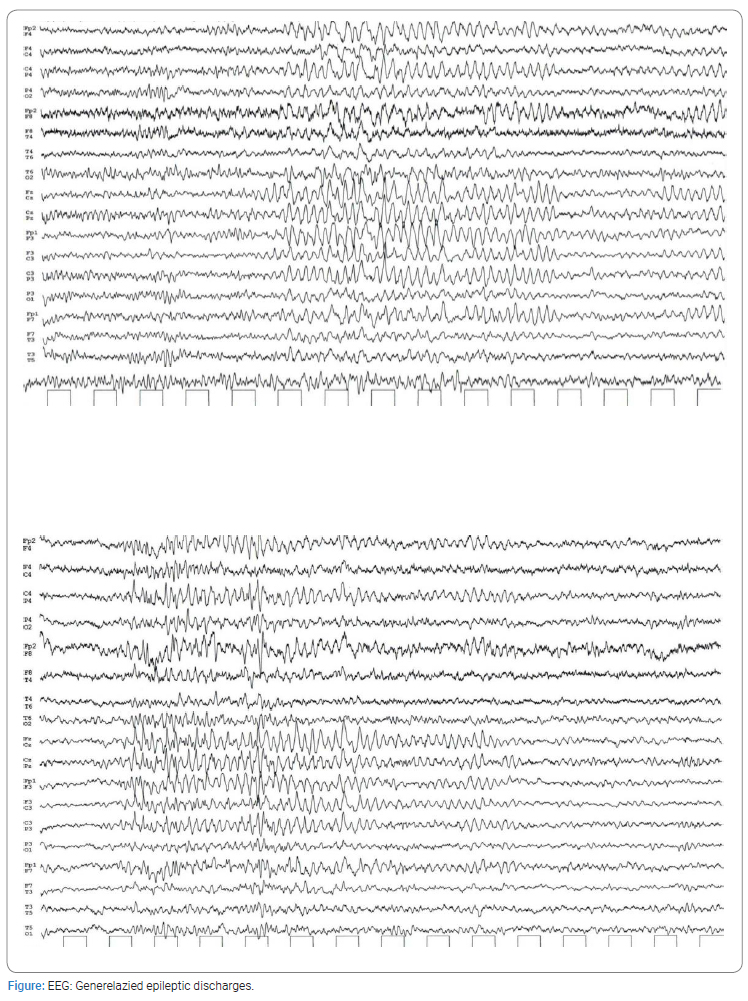
The Cerebrospinal Fluid (CSF) examination revealed elevated white blood cell count (10 cells/μL) with lymphocytic predominance (90%), normal protein, and negative bacteriological cultures. Serum and CSF tests for viruses, bacteria, tuberculosis, syphilis, West Nile Virus (WNV), and encephalitis were all negative (Table). The Electroencephalography (EEG) revealed generalized spike and slow wave discharges (Figure). Anticonvulsant treatment with levetiracetam 500 mg twice daily was initiated, and the symptoms resolved after ten days. CSF analysis and the resolution of symptoms raised the diagnosis of DIAM.
Discussion
The number of patients treated with IFX is growing rapidly around the world. Over 2.8 million people were exposed to this drug from 1998 to 2018 [1]. A very rare but significant side effect of IFX is DIAM. A comprehensive search of PubMed/MEDLINE to identify studies reporting on IFX-induced meningitis revealed only nine previous cases. Six cases were reported in patients with Inflammatory Bowel Disease (IBD) [8], two with rheumatoid arthritis [3,9], and one with psoriatic arthritis [10]. The duration of therapy with IFX varies between cases. Most of them, including our case, were treated with IFX for less than a year before the onset of DIAM.
The time interval between drug exposure and DIAM symptoms ranged from a few hours up to two weeks [2–9]. Symptoms are headache and fever accompanied by neck stiffness, arthralgia, and myalgia. The resolution of symptoms ranged from one to two weeks.
The patient, in this case, reported having an acute reaction after each of the IFX infusions, with symptom resolution 48 hours post-infusion. According to reported cases, there are three more patients that described self-restricted adverse reactions after IFX infusion [4,5,8]. All patients had a milder acute reaction to an earlier dose that was compatible with a serum sickness reaction. In 2004, a large clinical trial described that 2.8% of patients had serum sickness reactions with IFX [10]. Moreover, the previously described cases were on co-treatment with steroids. In contrast with, our patient and did not develop DIAM until steroids were tapered or stopped. The contribution of steroids in preventing DIAM is debatable, but it can give us an idea of possible future treatment.
The diagnostic workup of DIAM requires infectious and other causes to be excluded. CSF analysis is crucial. A review of the literature showed that examination of CSF demonstrates neutrophil-predominant pleocytosis (10 cells/mm3–1000 cells/mm3), normal to low glucose, and increased protein. By way of exception, our case is the third in total, which is excluded from the average by having predominantly lymphocyte-type cells.
We are proposing the following criteria to be met for the diagnosis of DIAM: patients with underlying autoimmune disease (e.g. Inflammatory Bowel Disease), relevant medical history, CSF pleocytosis (> 5 cells/mm3), which can be neutrophil-predominant, negative cultures, MRI of the brain and whole-body Computed Tomography (CT) scan for the exclusion of paraneoplastic syndrome, negative autoimmunity-related antibodies, resolution of symptoms on drug withdrawal and absence of any other explanation. Moreover, our case introduces an additional criterion in the diagnosis of DIAM, the need for EEG. The above examination, despite the absence of symptoms, indicated pathological findings and mandated the administration of anti-convulsant prophylaxis.
The exact pathophysiological mechanism of DIAM is unknown. The two proposed mechanisms are inhibition of TNF-α and hypersensitivity reaction to the drug [1]. In view of peripheral inhibition of TNF, it could enhance the action of brain-derived TNF-α and lead to side effects in the central nervous system. Alternatively, DIAM may be related to an atypical form of an immune complex-derived allergic reaction (type III reaction) as it occurs several days to weeks after the administration of the drug. In light of this hypothesis, serum sickness reactions are reported in one-fourth of patients who received infliximab after at least a 2-year treatment interval, and affected patients develop high titers of anti-infliximab antibodies [1]. Most likely, then, the mechanism of serum sickness is hypothesized to be the prevailing one.
Conclusion
This case calls attention to a rare and unusual occurrence of aseptic meningitis in a patient on anti-TNFα. Initially, our case emerges a rare observation that CSF analysis demonstrates lymphocytic-predominant pleocytosis. Given the key role of tumor necrosis factor in the innate immune response, patients receiving treatment with IFX are probably susceptible to neurological diseases with a lymphocytic type of CSF like Mycobacterium tuberculosis, Syphilis, Viral Encephalitis (WNV, Herpes Simplex Virus) and Malignancies-Lymphomas. On close analysis and appraisal, we highlight the need for the exclusion of previously mentioned diseases in order to diagnose DIAM. Moreover, we emphasize the importance of performing an EEG in every patient with DIAM suspicion and the administration of anticonvulsants for pathological findings. The fact that our patient appears to have serum sickness symptoms in response to prior infusions, without steroids co-therapy as in the mentioned cases, represents a part of the hypersensitivity phenomenon. Considering the perceived documents, the pathophysiology of infliximab-induced DIAM could be attributed to an immune-mediated process.
To conclude, we suggest that clinicians should be aware of IFX side effects. Headaches can go unrecognized if the clinician is not aware of the DIAM possibility. Given the possible implications in the clinical practice of IFX, administration by a maintenance regimen rather than episodic treatments, avoidance of prolonged drug holidays, and steroids as pretreatment are warranted.
Conflict of Interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Informed consent was obtained for this publication.
Keywords
Aseptic meningitis; Infliximab; Crohn’s disease
Cite this article
Stamatiou I, Tartanis G, Tsakaldimi S, Tsantsas C, Delivalta N, Ntoga M, et al. Infliximab induced aseptic meningitis during treatment of Crohn’s disease. Case report and review of literature. Clin Case Rep J. 2023;4(1):1–4.
Copyright
© 2023 Stamatiou Iliana. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-4.0).


