Abstract
The pathophysiology of renal dysfunction in COVID-19 infection is multifactorial and of high complexity. Myeloperoxidase-antibody-associated glomerulonephritis is a type of glomerulonephritis characterized as a pauci-immune type of necrotizing and crescentic GN. We report two patients who developed ANCA-associated glomerulonephritis shortly after a COVID-19 infection. Both patients, previously healthy and not known with autoimmune diseases, presented with an acute kidney injury after a COVID-19 infection. Lab results revealed a high anti-MPO titer and significant proteinuria. Renal biopsy was consistent with pauci-immune glomerulonephritis, and induction therapy with glucocorticoids and cyclophosphamide was started. There was a favorable evolution with complete recovery of renal function in the first patient and partial recovery in the second. Recent literature suggests an association between SARS-CoV2 and anti-MPO vasculitis based on the underlying pathogenesis. Therefore, a renal biopsy can play an essential role in differentiating the mechanism of COVID-19-associated kidney injury.
Introduction
Since the beginning of the COVID-19 pandemic in early 2020, there have been more than 500,000,000 confirmed cases, including 6,250,000 deaths [1]. Although pulmonary impairment is the most prevalent manifestation, the COVID-19 cytokine storm is known to affect multiple organs, including the kidneys [2]. Large observational studies revealed an incidence of up to 40% in hospitalized patients [3]. The pathophysiology of renal involvement is of high complexity [4]. Besides the indirect influences of hemodynamic changes and hypoxemia in the critical ill, direct changes such as cytokine release, endothelial injury, and activation of coagulation play important roles. Binding partners for the virus on the renal tubular cells have been described, but the role of renal viral tropism remains uncertain [4–6]. Renal biopsies learned that acute tubular injury is the most prevalent form of kidney damage in COVID-19, followed by a specific form of glomerular lesions in the population with APOL1 variant alleles, being a collapsing glomerulopathy [4,6].
In addition, it is well known that some viruses play a role in vasculitis (called secondary vasculitis). However, to the best of our knowledge, only a handful of cases have been described of COVID-19 induced crescent glomerulonephritis worldwide [7–14]. Here, we describe two cases of COVID-19-induced ANCA-associated glomerulonephritis.
Case Presentation
Case 1: A 70-year-old woman was referred because of an acute kidney injury in the absence of symptoms. The serum creatinine at that moment was 2.2 mg/dl with an eGFR of 22 ml/min (CKD-EPI), whereas the month before, there was a normal serum creatinine of 0.8 mg/dl with an eGFR of 68 ml/min (CKD-EPI). Subsequent urinary analysis revealed proteinuria of 2.05 g/g creatinine and positive sediment with leukocyturia (49/µl) and hematuria (615/µl).
Her medical history was brief, with a herpes zoster infection in 2021, SARS-CoV 2 infection two months before presentation, and pauci-symptomatic without fever or desaturation. In the weeks following her COVID-19 infection, there was increased fatigue, dyspnea, and anorexia. She lost 5 kg unintentionally, despite a good fluid intake of 1.5 L/day.
Medication was limited to the occasional use of Acetaminophen. There was no use of non-steroidal anti-inflammatory drugs.
At the time of the first presentation at our clinic, the patient was completely asymptomatic. On examination, blood pressure was significantly elevated (160/111 mmHg), with a heart rate of 90/min. The patient appeared euvolemic without clinical signs of dehydration. Heart and lung auscultation were normal, and there was an absence of edema or skin rash.
A diagnostic work-up with an echography of the kidneys and renal vasculature proved reassuring, with a normal bipolar diameter of 12 cm and normal vascular flow. A urinary sample revealed proteinuria of 2.7 (P/C ratio) with positive sediment. Protein electrophoresis on the urine and blood was normal. Viral serology for hepatitis B, C, and HIV was negative. The other lab values were reassuring without signs of inflammation (no leukocytosis, CRP 5 mg/l) (Table 1). ANCA-antibodies were positive with a high titer for anti-myeloperoxidase (anti-MPO antibodies).
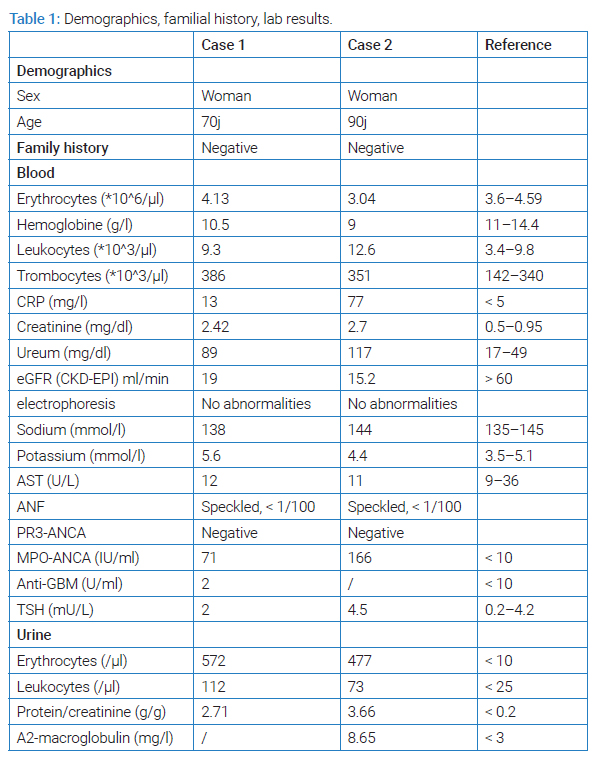
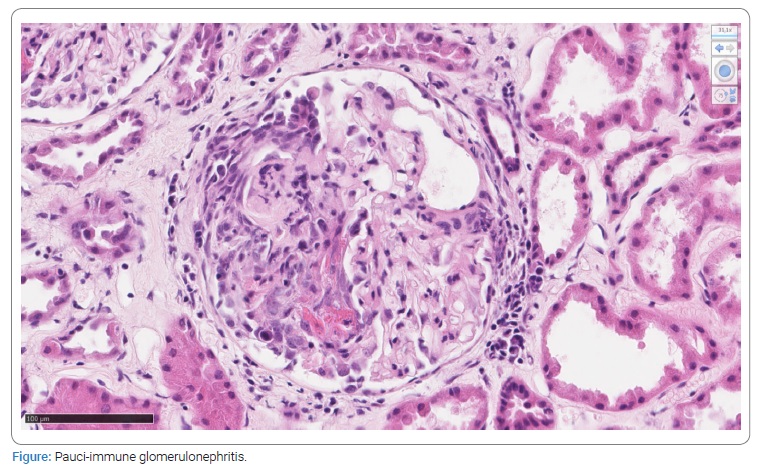
A renal biopsy was performed, which showed crescentic pauci-immune glomerulonephritis, consistent with acute ANCA-mediated glomerulonephritis (Figure).
Combination therapy with methyl-prednisolone and cyclophosphamide was started.
There was a favorable evolution with complete recovery of renal function (serum creatinine < 1 mg/dl) and disappearance of proteinuria.
Case 2: A 90-year-old patient was hospitalized for three days at the end of March 2022 because of a fever. There was some flank pain and moderate shortness of breath. She had no chest pain or palpitations. There was no dysuria. There was no skin rash or arthralgia. Her intake was preserved. Her medical history was limited for her age: a radical hysterectomy in 1974, paroxysmal atrial fibrillation, and an occluded Posterior Descending Coronary Artery (PDA).
On first examination, the patient had marked arterial hypertension of 170/80 mmHg with preserved oxygen saturation at rest (97% at ambient air). On auscultation, the patient had crepitations right basally, and there were bilateral pitting edemas. Chest radiography revealed moderate pulmonary congestion. SARS-CoV-2 Reverse-Transcriptase Polymerase Chain Reaction (RT-PCR) on a nasal swab was positive.
There was a significant amount of inflammation with a CRP of 107 mg/l and an increased sedimentation of 42 mm/h. The serum creatinine was elevated (2.1 mg/dl, compared with a baseline value of 1.05 mg/dl) with no electrolyte abnormalities (Table 1). Urinary examination revealed important hematuria (363/µl) and leukocyturia (508/µl) with proteinuria of 2.2 g (P/C ratio). Urine culture was positive for Escherichia coli.
The diagnoses of a urinary tract infection with E. coli, a SARS-CoV2 infection, and an acute kidney injury were made. Despite antibiotic treatment (Levofloxacin) and intravenous fluid, there was an increase in inflammatory markers and a decrease in kidney function (Table 1). The echography of the kidney was reassuring. An autoimmune screening was performed. There was a sharply increased titer for anti-MPO antibodies (ANCA). The tentative diagnosis of MPO-associated glomerulonephritis was made.
Because of the age and severe arterial hypertension, we decided not to perform a renal biopsy. Corticosteroids and Cyclophosphamide were started.
The immunosuppressive therapy had to be reduced due to side effects. We saw only a partial recovery of renal function.
Discussion
We present two cases of myeloperoxidase-antibody-associated crescentic glomerulonephritis (GN). One patient developed the GN during her COVID-19 infection, and the other patient after a latent period of 7 weeks. Both patients were not known with autoimmune diseases prior to their Covid-infection.
Last year, Ferlicot et al. released a multicentric retrospective case series of 47 kidney biopsies in Covid-associated acute kidney injury. A prevalent form of glomerular injury (36%) was collapsing glomerulopathy, in this context designated as ‘COVAN’ [6,15,16]. Similar results were found by Akilesh et. al [5,17]. No pauci-immune crescentic glomerulonephritis was observed in those trials.
Nevertheless, a recent series of case reports described vasculitis-associated glomerulonephritis during/ after a recent COVID-19 infection (Table 2). The question arises whether there’s a true causal association. Considering the high incidence of COVID-19 between 2020 and the present (14-day incidence of COVID-19 at the time of writing was 1156/100 000 p.) [18], a coincident association is possible.
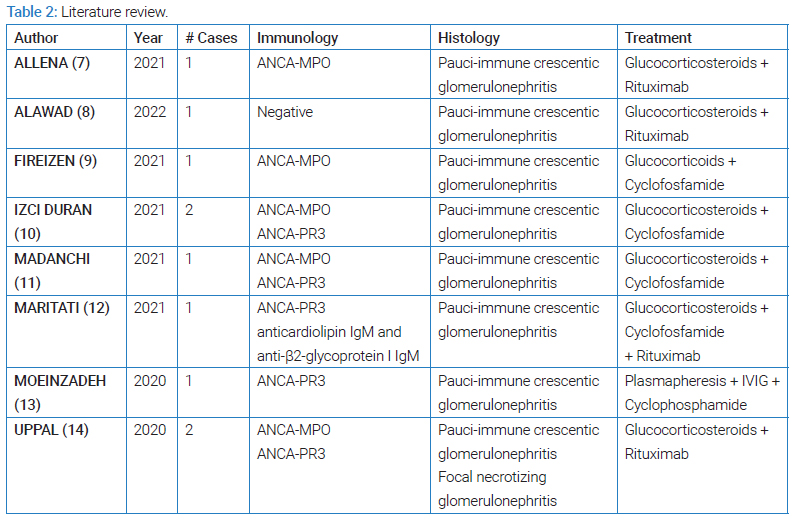
However, we suspect that causality might be the case when we take a deeper look at the underlying pathogenesis. It’s known that SARS-CoV-2 can provoke a cytokine storm, which dysregulates the adaptive immune system and increases the risk of auto-antibodies formations.
Becker et al. recently reviewed the immune function of microvascular endothelial cells and their response to COVID-19 injury. They showed a direct toxic effect of cytokines resulting in endothelial cell injury and neutrophil activation [19]. In COVID-19, the Neutrophil-to-Lymphocyte Ratio (NLR) is increased and correlates directly with the severity of COVID-19. Neutrophils can produce extracellular traps (NETs), which play a central role in the immune response to COVID-19. As recently reviewed by Al-Kuraishy et al. SARS-CoV-2 can trigger the formation of NETs by cytokine release, ROS formation, and downregulation of ACE2, which inhibits neutrophil infiltration [20]. Myeloperoxidase is part of those NETs [19,20]. It’s presumed that NETs serve as a source of autoantigens presenting MPO to the immune system in genetically susceptible individuals. Multiple autoimmune disorders provoked by COVID-19 or vaccines have been described, as reported by Kronbichler et al. [21].
In our patients, induction therapy with glucocorticoids and cyclophosphamide was preferred over rituximab because of the additional risk of the latter, as described in the literature [22]. However, despite the possible additional risk, the importance of adequate immunosuppressive therapy has been stated by Bruchfeld et al. [22].
The two cases (along with a recent series of case reports) demonstrate the importance of further investigations into a COVID-19-associated acute kidney injury. If an underlying glomerulonephritis is suspected, a renal biopsy can be an important diagnostic tool to elucidate the underlying pathophysiology and initiate correct therapy.
More (fundamental) research is required to explore the relationship between SARS-CoV-2 and glomerulonephritis further.
Conflict of Interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Informed consent was obtained for this publication.
Keywords
ANCA/Pauci-immune glomerulonephritis; Covid-19; Renal biopsy
Cite this article
Thomas VO, Jan D, Dendooven A, Valerie N. ANCA-associated glomerulonephritis post COVID-19 infection: Two case reports and review of the literature. Clin Case Rep J. 2023;4(2):1–5.
Copyright
© 2023 Van Overmeiren Thomas. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-4.0).



