Lipschu¨tz´s Acute Vulvar Ulcer: A Case Report
Abril Camacho-Cervantes;
* Itzel Guadalupe Castillo-Duarte;
Sheila De la Cruz-Aragón;
Jorge Galvez-Muñoz;
-
Abril Camacho-Cervantes: Department of Obstetrics and Gynaecology, Hospital Español, Ciudad de México, México.
-
* Itzel Guadalupe Castillo-Duarte: Department of Obstetrics and Gynaecology, Hospital Español, Ciudad de México, México; Universidad Nacional Autónoma de México, Ciudad de México, México.
-
Sheila De la Cruz-Aragón: Department of Obstetrics and Gynaecology, Hospital Español, Ciudad de México, México; Universidad Nacional Autónoma de México, Ciudad de México, México.
-
Jorge Galvez-Muñoz: Department of Obstetrics and Gynaecology, Hospital Español, Ciudad de México, México.
Abstract
In 1913, Lipschütz’s ulcers (acute genital ulcers nonsexually related), also known as ulcus vulvae acutum, were originally described by Dr. Benjamin Lipschütz. The etiology still needs to be fully clarified, and in 70% of the cases, it is classified as idiopathic. It is known that these ulcers have a predilection for the labia minora but can also be present on the labia majora or in the perineal region. Vulvar acute ulcers have been mainly associated with viral infections, such as Epstein-Barr virus, cytomegalovirus, influenza A and B, parvovirus B19, and adenovirus, among other infectious agents. In the last couple of years, it has also been associated with SARS-CoV-2 infection or vaccination against SARS-CoV-2, suggesting that the pathogenesis is associated with a related infection that triggers the humoral immune response.
The case report literature on Lipschütz’s acute vulvar ulcer is scarce, and therefore, the condition is considered to be underdiagnosed. The aim of this article is to present a case of Lipschütz’s ulcer in its miliary variant in a 10-year-old patient and analyze the clinical features, underlying causes, diagnostic criteria, disease duration, main differential diagnoses, and recommended treatment.
Conclusion: Lipschütz ulcer is a rare entity often underdiagnosed, presenting as painful vulvar ulcers. Despite the few cases reported in the literature, its acute course and spontaneous resolution are well-established. The main differential diagnoses include sexually transmitted infections and certain systemic diseases. Misdiagnosis can lead to the unnecessary use of treatments and poor treatment adherence.
Introduction
In 1913, Lipschütz’s ulcers (acute genital ulcers nonsexually related), also known as ulcus vulvae acutum, were originally described by the Jewish-Austrian dermatologist and microbiologist (virologist), Dr. Benjamin Lipschütz [1]. Later, in 1984, Portnoy et al. described a relationship between these ulcers and Epstein-Barr virus [2]. However, other viral infections such as cytomegalovirus, influenza A and B viruses, adenovirus, rubeola, COVID-19, Salmonella paratyphi, Mycoplasma pneumoniae, and Borrelia burgdorferi have also been associated with them [3]. These genital ulcers have an acute onset, are characterized by being painful, and appear in the pediatric and young women population, generally with no history of sexual initiation. The diagnosis is made by exclusion [4–7].
The etiology still needs to be fully clarified. However, it is known that these ulcers have a predilection for the labia minora and can also be present on the labia majora or in the perineal region [5–7]. Typically, they present as a single deep ulcerated lesion that is sharply demarcated, painful, and marked by fibrinous coatings or a greyish exudate [5,8]. Lipschütz’s ulcers mostly evolve favorably and go into spontaneous remission [9].
The most accepted hypothesis regarding its pathogenesis is a type 3 hypersensitivity reaction. In the first phase, cytolysis of infected cells occurs, followed by the deposition of immune complexes, complement activation, recruitment of neutrophils, and other cells of the immune system. This process leads to the formation of microthrombi, which, in turn, produce deep, necrotizing, and painful aphthous ulcers [10,11].
The case report literature on Lipschütz’s acute vulvar ulcer is limited; therefore, the condition is considered underdiagnosed [12–14]. The aim of this article is to present a case of Lipschütz’s ulcer in its miliary variant in a 10-year-old patient and to discuss the clinical characteristics and main differential diagnoses. By improving the recognition of this type of ulcer, we can offer timely diagnosis and treatment to patients, thus avoiding the use of unnecessary treatments, poor adherence to them, and unfavorable outcomes.
Case Presentation
A 10-year-old female patient presented to the emergency department of our hospital with a 2-week history of fever, as well as severe genital pain due to a superficial vulvar ulcer with a necrotic border on the left labia majora, which had been present for five days (Figure 1), along with a non-fetid yellow vaginal discharge. The patient had sought care in different emergency services in the previous days and had been treated with various antibiotic and antiviral regimens, but there was no improvement in the symptoms.

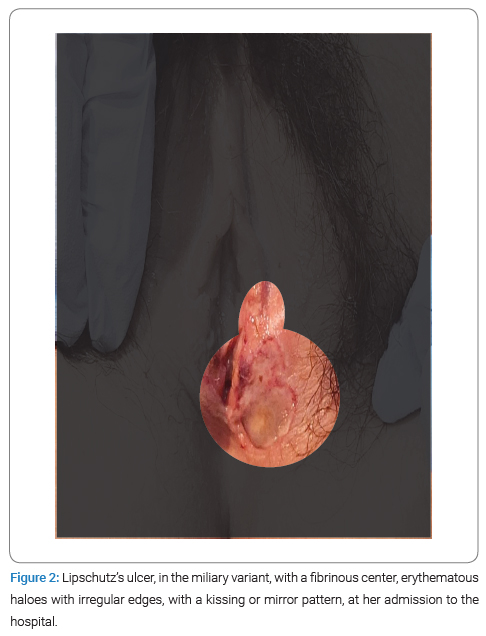
She had a medical history of Hepatitis A virus infection one month prior to admission and multiple urinary tract infections. Menarche occurred at nine years of age, with regular menstrual cycles. She had not initiated her sexual life and denied previous surgical intervention. On physical examination, a 1.5 cm diameter ulcerative lesion was observed in the lower third of the left labia majora (Figure 2) with irregular borders, an erythematous halo, the fundus containing fibrinoid-like material extending to the vaginal introitus. There was also the presence of a contralateral ulcer with the same characteristics, exhibiting a symmetric appearance known as the “kissing phenomenon.” These ulcers were painful on palpation, and no palpable lymphadenopathy was detected.
At the time of the patient’s admission to the hospital, a COVID-19 test was administered, yielding a negative result. The complete blood count and blood chemistry were within normal parameters. Acute phase reactants were measured, with the following results: PCR 7.96 mg/dL, ESR 46 mm/h, and Procalcitonin 0.15 ng/mL. General urinalysis and urine culture showed negative results. Anti-Herpes Simplex Virus type 1 and 2 IgG and IgM antibodies and a CMV test were requested and returned negative results. Anti-Epstein Barr IgG antibodies were reactive with non-reactive IgM. Bacteriological and sexually transmitted disease cultures of the genital ulcer were negative, ruling out syphilis or HIV infections.
We established a diagnosis of Lipschütz’s ulcer with all the collected information. It was decided to treat her with a topical steroid, antibiotic ointment, and mild painkillers, as her systemic inflammation was not severe. The ulcer gradually improved over the next ten days, and she did not require further treatment.
Discussion
Ulcus vulvae acutum Lipschütz (UVAL) is a painful ulcer of unknown etiology with an acute onset that primarily occurs in nonsexually active adolescent females [6,14–16]. These ulcers typically manifest with a prodromal phase of viral symptoms, including fever, fatigue, and malaise, followed by single or multiple vulvar ulcerations ranging from 0.1 cm to 2.5 cm in size [17]. According to Beiro-Felipe et al. (2012), Lipschütz’s ulcers can occur in two clinical variants, depending on their clinical course and morphology: 1. Gangrenous: This is the most frequent presentation and is characterized by hyperacute, deep ulcers with a white-gray background that eventually disappear, leaving a scar. It is prone to recurrence and is often associated with systemic symptoms. 2. Miliary: These ulcers are fibrinous, superficial, purulent, and small in size (approximately 1 cm). They are not usually associated with systemic symptoms and typically heal without sequelae or recurrence, as in the case we present here [18].
Regarding the prevalence, it remains uncertain. There are few case reports and case series reported in the literature. In a systematic review, Vismara SA et al. (2020) suggested that approximately one out of three patients presenting with an acute onset of vulvar ulcers is related to Lipschütz’s vulvar ulceration [19]. The etiology of Lipschütz’s ulcers remains unknown, and in 70% of cases, it is classified as idiopathic [4]. Vulvar acute ulcers have been mainly associated with viral infections such as Epstein-Barr virus, cytomegalovirus, influenza A and B viruses, parvovirus B19, adenovirus, or infective agents such as Toxoplasma gondii, Salmonella paratyphi, Ureaplasma, Mycoplasma pneumoniae, Group A streptococcus, Borrelia burgdorferi, and Lyme disease [3,4,9,10,21]. In the last couple of years, it has also been associated with SARS-CoV-2 infection or vaccination against SARS-CoV-2, but only if other more likely causes have been excluded [3,9,20].
Its clinical features form the basis for diagnosis, but it should be made by exclusion [20–23]. The ulcers typically present with hyperalgesia, well-defined edges, a necrotic, fibrinous, or purulent base, a mirror-like vulvar distribution, and acute evolution. They usually appear in the vestibule and labia minora. They can be associated with regional lymphadenopathy, frequently preceded by a flu-like syndrome, and tend to resolve in less than three weeks [20]. It is also very important to consider that in the clinical scenario of genital ulcers, always keep in mind that sexual abuse should be ruled out [13].
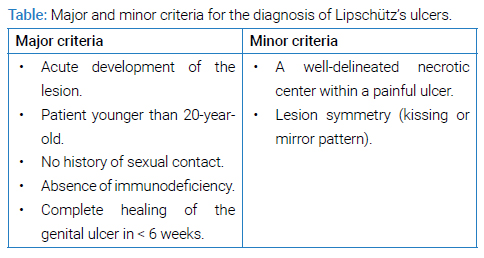
In 2017, diagnostic criteria for Lipschütz ulcer were established, with the exclusion of sexually transmitted infections. Both major and minor diagnostic criteria must be considered, and all major criteria and at least one minor criterion should be fulfilled (Table) [4,10,24]. In our case, all the major and minor criteria were met, venereal infections were ruled out, the necessary additional tests were conducted to rule out differential diagnoses, and since the clinical course was favorable, the diagnosis of Lipschütz’s acute vulvar ulcer was justified.
Differential diagnoses of Lipschütz’s ulcer include acute genital ulcers with infectious or venereal origins (syphilis, herpes simplex virus, Lymphogranuloma venereum, and chancroid), non-venereal infectious origins (cytomegalovirus and Brucella), or non-infectious origins (Behçet’s disease, Crohn’s disease, Pemphigus vulgaris, and drug eruption) [10]. Considering these ulcers typically resolve in less than three weeks, the recommended treatment is based on reassurance, local hygiene, wound care, and pain control as the mainstay for managing this benign and self-remitting condition [19,24]. Systemic corticosteroids are controversial, so they are reserved for the most severe cases with significant inflammation [25]. It is important to highlight that these lesions have been associated with the subsequent development of autoimmune diseases, such as Sjögren’s syndrome; therefore, monitoring and follow-up are recommended [4,24].
Conclusion
Lipschütz’s ulcer is a rare condition that is often underdiagnosed. Despite the limited number of cases reported in the literature, its acute course and spontaneous resolution are well-established. Understanding the complementary tests and differential diagnosis enables a better approach to the patient, offering the opportunity for timely diagnosis and appropriate treatment. Misdiagnosis can result in the unnecessary use of treatments and poor treatment adherence.
Conflict of Interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Informed consent was obtained for this publication.
References
- Lipschütz B. Über eine eigenartige Geschwürsform des weiblichen Genitales (ulcus vulvae acutum). Archiv für Dermatologie und Syphilis. 1913;114:363–395.
- Portnoy J, Ahronheim GA, Ghibu F, Clecner B, Joncas JH. Recovery of Epstein-Barr virus from genital ulcers. N Engl J Med. 1984;311(15):966–968.
- Gru AA, Plaza JA, Sanches JA, Miyashiro D, Sangueza OP, Puccio FB, et al. An update on Epstein-Barr virus-and human T-lymphotropic virus type-1-induced cutaneous manifestations. CME Part II. J Am Acad Dermatol. 2023;88(5):983–998.
- Luque-González P, Azcona-Sutil L, Vargas-Gálvez D, Carmona-Domínguez E, Barroso-Tudela C, Cabezas-Palacios MN. Úlcera de Lipschütz: reporte de un caso y revisión bibliográfica. Ginecol Obstet Mex. 2020;88(9):644–650.
- Huppert JS. Lipschutz ulcers: evaluation and management of acute genital ulcers in women. Dermatol Ther. 2010;23(5):533–540.
- Vieira-Baptista P, Lima-Silva J, Beires J, Martinez-de-Oliveira J. Lipschütz ulcers: should we rethink this? An analysis of 33 cases. Eur J Obstet Gynecol Reprod Biol. 2016;198:149–152.
- Chanal J, Carlotti A, Laude H, Wallet-Faber N, Avril MF, Dupin N. Lipschütz genital ulceration associated with mumps. Dermatology (Basel, Switzerland). 2010;221(4):292–295.
- Svedman C, Holst R, Johnsson A. Ulcus vulvae acutum, a rare diagnosis to keep in mind. Eur J Obstet Gynecol Reprod Biol. 2004;115(1):104–105.
- Sangster-Carrasco L, Paz-Temoche R, Coronado-Arroyo J, Concepción-Zavaleta M, Roseboom P, Concepción-Urteaga L, et al. Lipschütz acute vulvar ulcer related to COVID-19 vaccination: First case report in South America. Medwave. 2023;23(2):2674.
- Miyazawa T, Hayashibe R, Nozawa T, Nishimura K, Ito S. Lipschütz ulcer induced by acute Epstein-Barr virus infection in a young girl. Pediatr Int. 2022;64(1):e15022.
- Schmitt TM, Devries J, Ohns MJ. Lipschutz ulcers in an adolescent after Sars-CoV-2 infection. J Pediatr Health Care. 2023;37(1):63–66.
- Levy Bencheton A, Agostini A, Mortier I, Sadoun C, Gamerre M. Ulcère de Lipschütz, un diagnostic méconnu [Acute vulvar ulcer of Lipschütz: a misdiagnosis entity]. Gynecol Obstet Fertil. 2011;39(3):e58–e60.
- Hueto-Najarro A, González-García G, Breton-Hernández P, Zarate-Tejero I, Lanuza-Arcos R, Ferrer-Santos P. [Lipschütz ulcers: a pediatric case report]. Arch Argent Pediatr. 2017;115(6):e436–e439.
- Sadoghi B, Stary G, Wolf P, Komericki P. Ulcus vulvae acutum Lipschütz: a systematic literature review and a diagnostic and therapeutic algorithm. J Eur Acad Dermatol Venereol. 2020;34(7):1432–1439.
- Delgado-García S, Palacios-Marqués A, Martínez-Escoriza JC, Martín-Bayón TA. Acute genital ulcers. BMJ Case Rep. 2014:2014:bcr2013202504.
- Hernández-Núñez A, Córdoba S, Romero-Maté A, Miñano R, Sanz T, Borbujo J. Lipschütz [corrected] ulcers--four cases. Pediatr Dermatol. 2008;25(3):364–367.
- Pereira DAG, Teixeira EPP, Lopes ACM, Sarmento RJP, Lopes APC. Lipschütz ulcer: an unusual diagnosis that should not be neglected. Rev Bras Ginecol Obstet. 2021;43(5):414-416.
- Felipe EB, Conde JC, Miguélez JL, Rubio GG, Ortiz DA, Valverde ML. Úlcera genital aguda en adolescentes. Úlcera de Lipschütz. Progresos de obstetricia y ginecología. 2012;55(4):193–195.
- Vismara SA, Lava SAG, Kottanattu L, Simonetti GD, Zgraggen L, Clericetti CM, et al. Lipschütz’s acute vulvar ulcer: a systematic review. Eur J Pediatr. 2020;179(10):1559–1567.
- Morais ML, Moura M, Moreira A. Lipschütz’s ulcers in an adolescent with SARS-CoV2 infection. Clin Case Rep. 2022;10(10):e6503.
- Charamanta M, Soldatou A, Michala L. Vulvar ulcers in children: dramatic but self-limited. Pediatr emerg care. 2021;37(2):70–72.
- Evangelio-Llorca B, Millet-Serrano S, Leal-Benavent A, García-Maset L. Úlcera de lipschütz. Prog Obstet Gynecol. 2008;51(7):438–444.
- Näher H, Gissmann L, Freese UK, Petzoldt D, Helfrich S. Subclinical Epstein-Barr virus infection of both the male and female genital tract--indication for sexual transmission. J Invest Dermatol. 1992;98(5):791–793.
- Arellano J, Fuentes E, Moreno P, Corredoira Y. [Ulcer of Lipschütz, a diagnosis to consider in the pediatric population]. Arch Argent Pediatr. 20191;117(3):e305–e308.
- González-Romero N, Morillo-Montañes V, Vicente-Sánchez I, García-García M. Úlceras de lipschütz tras la vacuna frente a la COVID-19 de astrazeneca. ACTAS Dermo-Sifiliográficas. 2022;113:S29–S31.
Keywords
Lipschütz; Non-sexually transmitted vulvar ulcers; Lipschütz’s ulcers; Vulvar ulcer; Genital ulcer
Cite this article
Camacho-Cervantes A, Castillo-Duarte IG, De la Cruz-Aragón S, Galvez-Muñoz J. Lipschü tz´s acute vulvar ulcer: a case report. Clin Case Rep J. 2023;4(4):1–4.
Copyright
© 2023 Itzel Guadalupe Castillo-Duarte. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-4.0).



