Abstract
Hypersensitivity rashes caused by Cystic Fibrosis Transmembrane Regulator (CFTR) modulators are rare, and there is no standard protocol on how to reinitiate the drug after the reaction. A 47-year-old woman with Cystic Fibrosis (CF) developed a maculopapular rash with Elexacaftor/Tezacaftor/Ivacaftor (ELX/TEZ/IVA). Since ELX/TEZ/IVA therapy produced a significant clinical response in the patient, and there is no other therapy that is as effective, we decided to use a slow desensitization protocol. We were able to restart the therapy effectively. There needs to be more data in the literature regarding the management of late drug reactions with ELX/TEZ/IVA and effective desensitization to ELX/TEZ/IVA. Herein, we shared our experience with desensitization to ELX/TEZ/IVA. After desensitization, our patient was able to proceed with her treatment without any issues.
Introduction
Cystic Fibrosis (CF) is an inherited disease resulting from a mutation in the gene encoding the cystic fibrosis transmembrane regulator (CFTR) protein [1]. CFTR protein acts as a chlorine channel in the epithelial cell membrane and regulates the transport of other ions. Although it is present in many cells, the respiratory and gastrointestinal system cells are mostly affected by its dysfunction. Up until recently, the primary objective of therapy was to relieve symptoms, but more recently, modulatory therapies—which target the underlying pathophysiologic mechanisms—have offered hope. Ivacaftor, Lumacaftor/Ivacaftor, Tezacaftor/Ivacaftor, and Elexacaftor/Tezacaftor/Ivacaftor (ELX/TEZ/IVA) have been approved for use in patients carrying at least one of the specific CFTR mutations. The treatment improves pulmonary function, reduces sweat test levels, and reduces deaths from pulmonary causes, thus improving survival.
ELX/TEZ/IVA is well tolerated in most patients. However, a proportion of patients treated with ELX/TEZ/IVA may experience drug-induced maculopapular rash compared with other CFTR modulators [2]. Drug discontinuation may be required depending on the extent and severity of the rash. With the discontinuation of ELX/TEZ/IVA, patients could not receive the clinically beneficial effects of the medication, which could lower their quality of life and have a negative impact on mortality and morbidity rates. Nevertheless, there is limited data on restarting the drug after a rash with CFTR modulators. Herein, we described a successful desensitization of a patient who developed maculopapular rash after initiating ELX/TEZ/IVA treatment.
Case Presentation
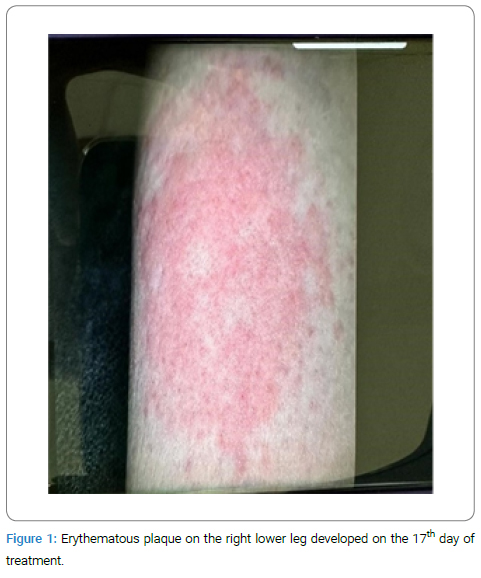
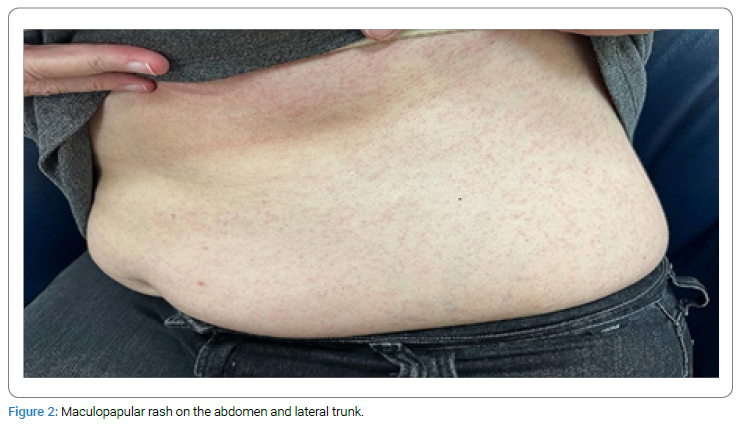
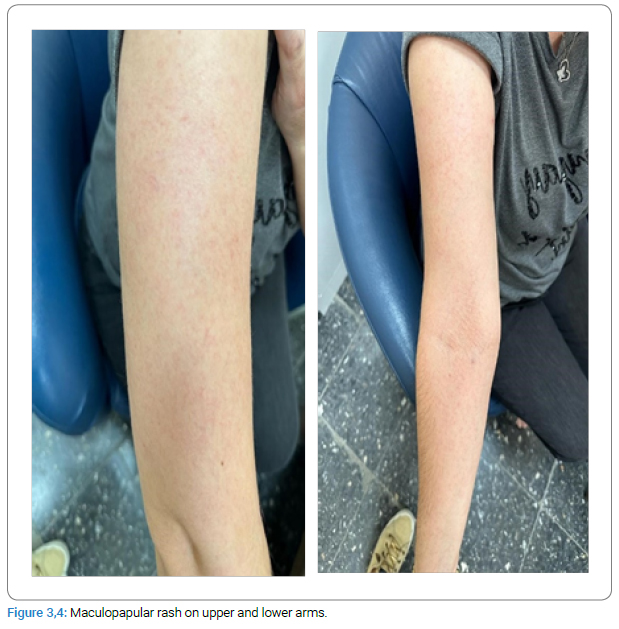
A 47-year-old woman, housewife, who was diagnosed with CF (p.G542X and p.H1054D heterozygous gene mutation positive) 20 years ago was started on Trikafta [Elexacaftor 100/Tezacaftor 50/Ivacaftor 75 mg (orange tablet) in the morning and 1 Ivacaftor 100 mg (blue tablet) in the evening] about one month ago. She first developed a pruritic, erythematous lesion (Figure 1) on the right leg 6 hours after taking the morning dose on the 17th day of the drug. The treatment with ELX/TEZ/IVA was continued, and topical beclometasone cream (twice a day) was started for the lesion, and it regressed within two days. A week later, non-pruritic swelling and redness (Figure 2–Figure 4) developed on the legs and spread to the abdomen and arms 4 hours after taking the morning dose, and thus, she consulted our allergy clinic. On presentation to our outpatient clinic, there were maculopapular lesions on the trunk and arms that faded under pressure. There were no additional systemic symptoms, and the other system examinations were normal. She described anaphylaxis with Penicillin and Bactrim that occurred 20 years ago and angioedema of the tongue with Rosithromycin 18 years ago during pregnancy. A punch biopsy was performed from the lesion in the lumbar region with a pre-diagnosis of allergic drug reaction. ELX/TEZ/IVA treatment was discontinued, and treatment with topical Baclomethasone cream bid, Desloratadine tablets bid, po, and Methylprednisolone 0.5 mg/kg/day, po with a dose reduction scheme was started. Pathological examination revealed superficial perivascular dermatitis with eosinophils (compatible with maculopapular drug reaction). Therefore, the maculopapular rash was diagnosed with a delayed-type allergic reaction to ELX/TEZ/IVA.
We decided to use a slow desensitization protocol (Table 1) and reinitiate the ELX/TEZ/IVA treatment since the therapy produced a significant clinical response in the patient. There is no other therapy that is as effective in the treatment of CF disease today [3]. In (Table 2), the changes we made to the desensitization scheme are explained.
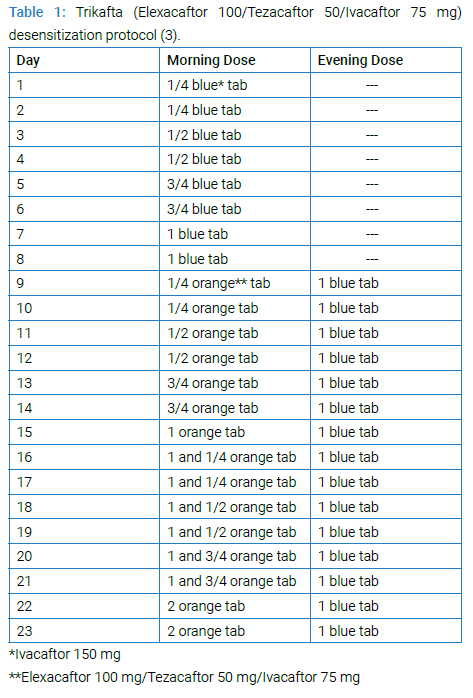
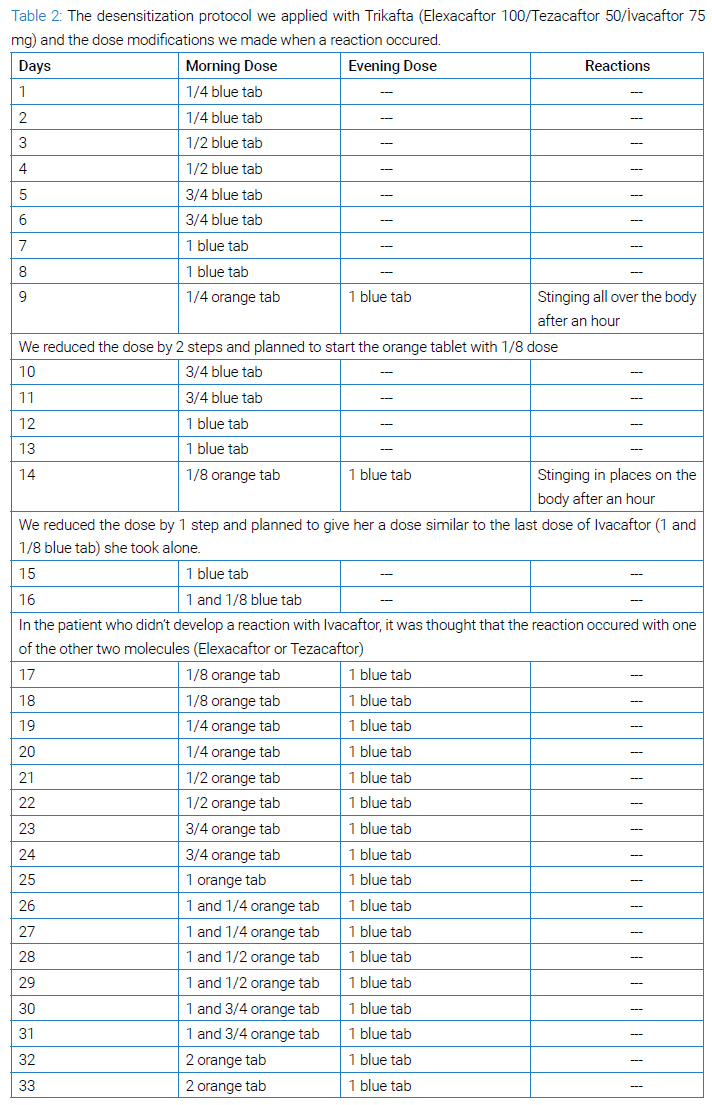
The protocol was started with ¼ blue tablet and increased to 1 blue tablet without any problem. In step 9, ¼ orange tablet was added in the morning, and one hour after taking one blue tablet in the evening, the patient developed a widespread stinging sensation in the whole body. It was deemed appropriate to return to the two-step previous doses (6th-day doses). The patient took the blue tablet steps without any reaction on days 10 to 13, and on day 14, the dose of orange tablet was reduced to 1/8 in the morning, and 1 hour after taking one blue tablet in the evening, a milder stinging sensation developed compared to the previous one. Thereupon, we reduced the dose by 1 step on day 15, and on day 16, she took 1+1/8 blue tablets to determine whether she could tolerate the increase in the total daily dose of Ivacaftor.
Thereafter, we thought that the reaction might have occurred with one of the other two molecules (Elexacaftor or Tezacaftor). Thus, the desensitization protocol was continued with 1/8 orange tablet and one blue tablet. (Table 2) summarizes all steps. The desensitization process was completed without any additional symptoms. The patient has been taking the full dose of the drug without any further reaction for the following six months after the desensitization protocol.
Discussion
Modulatory therapies in CF are well tolerated for most patients. However, with the widespread availability of drugs, the rate of drug-related hypersensitivity reactions (ADRs) is increasing. In a study of patients aged 12 years and older with the F508del mutation, approximately 11% (22/202 patients) reported erythematous, macular, or pruritic rashes compared to 6.5% (13/202 patients) in the placebo group [2]. In another study, in patients aged between 6 years and 11 years with the F508del mutation, 24% of patients (16/66 patients) reported maculopapular, itchy rash, or xerosis. In 8 (12%) of these patients, rashes were caused by ELX/TEZ/IVA, and one patient discontinued treatment [4]. The rash is more common in women, and co-usage of oral contraceptives increases the risk of an allergic reaction [2,5].
There is no consensus on the management of ADRs with CFTR modulators. Although the effectiveness of desensitization in type 1 IgE-mediated reactions is well-defined, there is no standard strategy for type 4 reactions. Both rapid or slow desensitization protocols can be used for maculopapular rashes. Given the efficacy of CFTR modulators in improving quality of life and reducing disease-related mortality and morbidity, it is of utmost importance to ensure that patients benefit as much as possible from the drug. Only few desensitization protocols with ELX/TEZ/IVA have been reported in the literature (ref ekle). After comprehensive discussion of the therapeutic options with patient, we decided to perform the desensitization protocol, and to re-start the medication since she had a significant benefit even in the first week of ELX/TEZ/IVA treatment and there was no alternative treatment. Following the desensitization, she continues to use her medication without any further reaction.
It is essential to pool as much as possible data in order to develop protocols with higher levels of evidence for the management of these patients. In the presence of a severe treatment-related rash, the drug should be discontinued immediately. The mechanism behind this immunologic adverse event is not clear and there is only one case report of a patient with ELX/TEZ/IVA-induced rash who recovered without drug discontinuation [6].
In conclusion, due to clinical heterogeneity, a uniform desensitization protocol for every ELX/TEZ/IVA associated cutaneous reaction is hardly possible. It is crucial to raise awareness and provide access to different titration schemes for the successful reintroduction of a life-changing treatment.
Conflict of Interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Informed consent was obtained for this publication.
Keywords
Necrotizing encephalitis; Genetics; Pediatrics; RANBP2 mutation; Neuroimaging
Cite this article
Çoban B, Ozturk RT, Ediger D. Successful Desensitization of Trikafta after Hypersensitivity Rash. Clin Case Rep J. 2024;5(2):1–5.
Copyright
© 2024 Burcu Çoban. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-4.0).





