Abstract
Pacemaker-associated endocarditis is a severe complication typically linked to Gram-positive organisms such as Staphylococcus and Streptococcus. We present a case of an active 59-year-old man with a Haemophilus parainfluenzae associated pacemaker lead infection, a rarely reported occurrence. The patient initially presented with fever, chills, and congestion, and subsequent blood cultures revealed the presence of gram-negative rods. Identification of H. parainfluenzae prompted further investigation due to the patient’s pre-existing pacemaker. Transthoracic echocardiography was inconclusive, but transesophageal echocardiography unveiled multiple large vegetations on the right atrial lead, ultimately necessitating pacemaker removal as part of the treatment regimen. To our knowledge, this is the first reported case of H. parainfluenzae infection of a pacemaker lead occurring in a man without significant risk factors.
Introduction
Over 400,000 Cardiac Implantable Electronic Devices (CIEDs) are implanted in the United States each year [1]. Infections in cardiac implants are serious complications, with an associated in-hospital mortality of 5%–15% [2]. Pacemaker-associated endocarditis is a severe complication typically linked to Gram-positive organisms like Staphylococcus and Streptococcus, with Gram-negative rods less commonly implicated [3–5]. The HACEK (Haemophilus spp., Aggregatibacter spp., Cardiobacterium spp., Eikenella spp., and Kingella spp.) group, identified in 1.5%–2% of all Infective Endocarditis (IE) cases [6], has rarely been associated with CIED infections. Its fastidious nature, characterized by slow growth in routine blood culture media, poses diagnostic challenges. H. parainfluenzae, an essential member of the HACEK group, has been reported in CIED infections, particularly pacemakers [4,7–10]. However, the focus on H. parainfluenzae as a causative agent in CIED-associated IE lacks momentum in the literature. We present a case of an otherwise healthy 59-year-old man with a pacemaker of 6 years who developed a pacemaker lead infection by H. parainfluenzae without recent pacemaker manipulation.
Case Presentation
A 59-year-old man with a history of sinus bradycardia with a Medtronic dual-chamber permanent pacemaker implanted six years ago presented to the Emergency Department (ED) with a one-week history of fever, chills, malaise, and nasal congestion. Two days earlier, he sought medical attention at the ED, where his white blood cell count was within normal limits, and a viral infection was suspected. Despite no antibiotic administration, two sets of blood cultures (2xBCX1) were obtained before discharge, revealing gram-negative rods. The patient was called back due to this finding, reporting persistent shortness of breath since his initial ED visit. Prior to this, he was generally healthy and exercised regularly despite having a pacemaker.
Upon examination, the patient’s vital signs were stable (T 36.70C, Pulse 76/min, RR 18/min, BP 104/62 mmHg, SPO2 99% on room air). The cardiopulmonary assessment revealed crackles at the right lung base, and the skin examination was negative for Osler’s nodes and Janeway lesions. Laboratory results showed leukocytosis (13.5 x 1,000/mcL), bandemia (14.0%), elevated ESR (35 mm/h) and CRP (25 mg/dL), Hyponatremia (sodium 128 mmol/L), elevated creatinine (2.5 mg/dL, baseline at 0.9 mg/dL), Transaminitis (ALP 368 U/L, AST 104 U/L, ALT 144 U/L), elevated Brain Natriuretic Peptide (BNP) (8,800 pg/mL), Troponin-I less than 0.012 ng/mL, and lactic acid (1.9 mmol/L). The Electrocardiogram (ECG) showed sinus rhythm with slight ST elevation in V2, V3, V4, I, and L without PR depression. A Computed Tomography (CT) chest without contrast showed no remarkable findings. A CT Pulmonary Embolism (PE) study did not proceed due to acute kidney injury.
With the identification of gram-negative rods, two repeat blood cultures (2xBCX2) were performed (Figure 1). The patient received empiric intravenous (IV) antibiotics, including cefepime (1 g once) and vancomycin (1.25 g once), and Lasix (40 mg IV once) was also given with concern of acute congestive heart failure at the ED, followed by admission for further management. Vancomycin was discontinued due to gram-negative bacteremia, and cefepime was continued at 1 g twice daily. Intravenous fluid was given the day after admission when acute heart failure was ruled out, and creatinine levels dropped to the baseline in 2 days, indicating acute kidney injury was resolved. Over the first two days of hospitalization, the elusive source of infection prompted investigations across the respiratory, gastrointestinal, and urinary systems, all yielding negative results. Suspecting endocarditis due to bacteremia with the presence of the pacemaker, a Transthoracic Echocardiogram (TTE) was performed. The TTE revealed normal left heart function with an ejection fraction of 55%–60%, and pacemaker leads in the right atrium and right ventricle without vegetation. However, it also indicated mild to moderate tricuspid regurgitation and mild pulmonary hypertension, with an estimated pulmonary artery systolic pressure of 38 mmHg. The first two sets of blood cultures (2xBCX1) returned positive for the gram-negative rod, H. parainfluenzae, beta-lactamase negative and susceptible to penicillin, after four days of culture, prompting an antibiotic switch from cefepime to ampicillin (2 g IV every 4 hours) following infectious disease recommendations. The second two sets of blood cultures on admission (2xBCX2) confirmed the same pathogen, while the third two sets of blood cultures (2xBCX3) obtained after two days of antibiotics showed no growth.
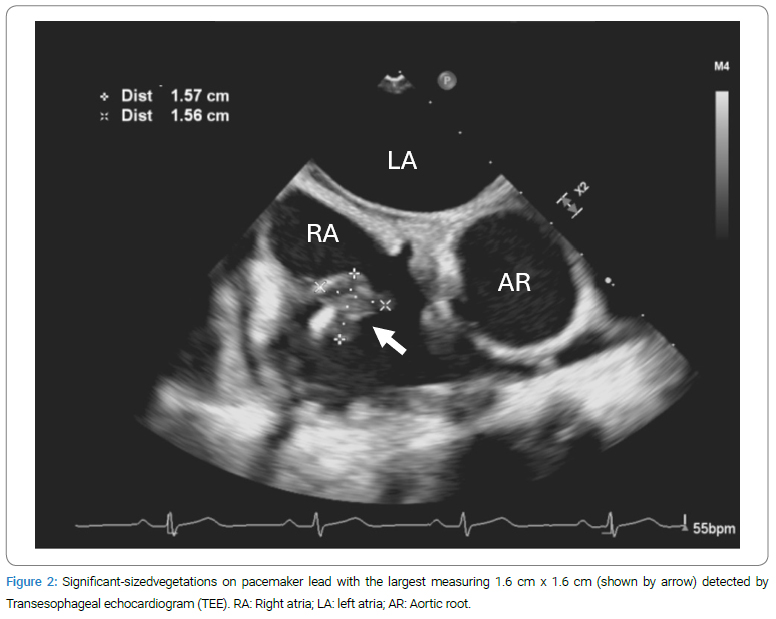
A Transesophageal Echocardiogram (TEE) proceeded and revealed significant-sized vegetations attached to the right atrial leads of the pacemaker, extending towards the Superior Vena Cava (SVC). The largest vegetation measured 1.6 cm x 1.6 cm (Figure 2), with mild flow obstruction at the SVC right atrium junction. Following cardiology recommendations, the patient was transferred to a nearby hospital for total pacemaker removal (day six from initial admission) with continued intravenous ampicillin administration at the same dose. During the procedure, a temporary transvenous pacemaker was inserted. The cultures of the extracted pacemaker leads showed no growth after three days. Two repeats of blood cultures that were obtained on day seven and day 9 (not shown in Figure 1) were both negative for growth. The temporary pacemaker was removed on day 9, demonstrating an uncomplicated procedure and a sinus rhythm of 60/min. A repeat TEE four days after the procedure (day 10) showed a resolving vegetation at the SVC-right atrium junction, with normal valve function and no evidence of valvular vegetations. The patient was asymptomatic cardiopulmonary-wise and continued with ampicillin treatment through a multidisciplinary decision-making process across infectious disease, cardiology, and electrophysiology.
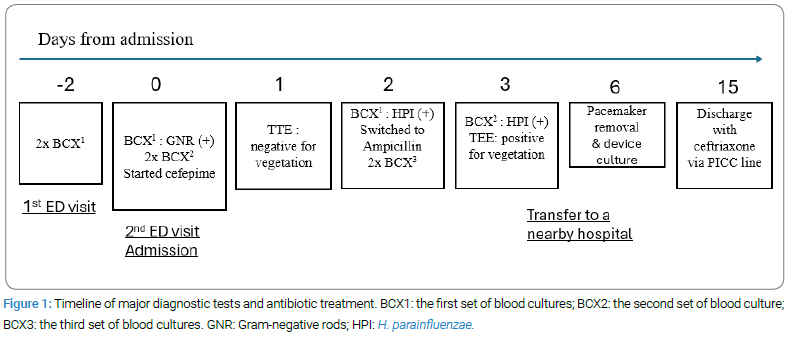
Upon discharge, the patient’s fever had resolved, and white blood cell counts normalized. Despite a heart rate in the 40 s/min, he demonstrated ambulatory capability and was discharged home on ceftriaxone 2 g once daily (up to a total of 6 weeks of antibiotic coverage from the first day with negative blood cultures) via intravenous infusion through a Peripherally Inserted Central Catheter (PICC) line. A follow-up echocardiogram two months after the initial presentation showed improvement in pulmonary hypertension with an estimated pulmonary systolic pressure of 27 mmHg. The patient remained functionally stable over the subsequent months. An outpatient electrophysiology study indicated possible sick sinus syndrome without debilitating symptoms. A 12-lead electrocardiogram in the office showed sinus bradycardia, a single blocked premature atrial contraction, first-degree atrioventricular block, borderline left ventricular hypertrophy, and likely an athletic heart. After careful reevaluation, the cardiac electrophysiologist determined that pacemaker reimplantation was not indicated. However, a dual-chamber leadless pacemaker should be considered if symptoms develop or syncope recurs. The patient is following up with infectious disease, cardiology, and electrophysiology on an outpatient basis.
Discussion
Although rare, H. parainfluenzae was the most common cause of HACEK endocarditis [11]. Our findings shed light on the rare occurrence of pacemaker-associated endocarditis caused by H. parainfluenzae, highlighting the emerging concept that such infections can occur in a healthy individual without traditional risk factors such as recent pacemaker manipulation or dental procedures. The exact mechanism of pacemaker-lead infection is unclear, but we suggest bloodstream entry, potentially through the upper respiratory tract. Initial symptoms of fever, chills, and nasal congestion support the hypothesis of a nasopharyngeal infection previously linked to H. parainfluenzae endocarditis [12,13]. H. parainfluenzae endocarditis typically manifests at a younger age, with a reported mean age range of 27–48 [11,14]. However, our patient, aged 59, highlights the possibility of occurrence at an older age. Despite our patient being generally healthy, his older age may be a risk factor for the progression from a previously asymptomatic persistence or colonization of H. parainfluenzae to the onset of clinical signs. Further evidence is needed to establish any potential association between age and this infection.
Diagnostic difficulties arose due to a delayed culture process, which could be 4 days–6 days. When blood cultures were positive, obtaining a Transthoracic Echocardiogram (TTE) and a Transesophageal Echocardiogram (TEE) proved necessary in assessing vegetation [15,16]. The diagnostic accuracy of TTE depends on the size of the vegetation and the underlying valvular disease. In native valve endocarditis, its sensitivity ranges from 40% to 63%, compared to TEE, which has a sensitivity of 90% to 100% [17]. In cases of CIED infections, TEE has significantly better sensitivity compared to TTE, with sensitivity rates of 90% versus 22%–43% [18]. A Positron Emission Tomography/Computed Tomography (PET/CT) study can be a reasonable option for identifying the source of infection when there is persistent bacteremia with inconclusive TTE/TEE findings [16]. Fortunately, in our case, a positive blood culture revealing gram-negative rods was obtained within about two days, which facilitated an immediate response from our emergency department, leading to the patient’s prompt evaluation. Despite initial suspicion of endocarditis related to the pacemaker, the diagnosis became elusive due to the initial uninformative TTE and the rarity of Gram-negative rods causing endocarditis. It was not until a subsequent TEE uncovered the presence of vegetation in the heart that the decision was made to remove the pacemaker completely.
Heart failure is the most common complication of infective endocarditis, occurring in 50% to 60% of cases [19]. A comprehensive 20-year literature review identified 39 adult cases of endocarditis attributed to H. parainfluenzae. In most cases, it affected the mitral valve with significantly sized vegetation (> 1 cm). Central nervous system septic embolization was common [20]. Our patient exhibited dyspnea alongside markedly elevated BNP and liver enzymes, suggesting a possible right-sided cardiopulmonary pathology in the context of pacemaker infection and bacteremia. This hypothesis was further supported by the detection of mild pulmonary hypertension on the initial echocardiogram, which improved following pacemaker removal. Such transient pulmonary hypertension likely resulted from septic pulmonary embolism frequently associated with Right-Sided Infective Endocarditis (RSIE) [21]. Due to the concurrent renal failure, a contrast CT chest was not pursued to confirm this possibility. However, it is important to emphasize that the absence of prompt treatment for H. parainfluenzae associated pacemaker infection poses a high risk of severe cardiopulmonary consequences.
While there is a lack of clinical trial data regarding the optimal choice and duration of antimicrobial therapy [16], our patient received 6 weeks of intravenous antibiotics from the first day with negative blood cultures. In addition to antibiotic treatment, early device removal has been associated with improved outcomes. Patients should be informed of the risks and benefits, as septic pulmonary embolism can occur when extracting pacemaker leads with large vegetations [22].
The estimated rate of pacemaker-associated infection rises from 0.77% for initial implants to 2.08% for revision or replacement procedures [23,24]. Notably, our patient did not necessitate pacemaker re-implantation following complete removal, underscoring the significance of judiciously considering pacemaker placement, particularly in the context of revision or replacement procedures [8,25]. According to the 2024 Scientific Statement from the American Heart Association, if a new device is indicated after reevaluation by the cardiac electrophysiologist, it is reasonable to use a Leadless Pacemaker (LPM) or a subcutaneous implantable cardioverter-defibrillator (S-ICD) to reduce the risk of re-infection [16].
Conclusion
This case underscores the rarity of Haemophilus parainfluenzae associated pacemaker-lead infection, emphasizing the concept that such infections can occur without traditional risk factors. It highlights the importance of a thorough diagnostic approach, including transesophageal echocardiography, and the significance of increased awareness of unusual pathogens in pacemaker-associated infections for prompt diagnosis and appropriate management, ultimately improving patient outcomes. Further research and documentation of similar cases are warranted to enhance our understanding of the clinical presentation and management of gram-negative organisms in pacemaker-associated endocarditis.
Conflict of Interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Informed consent was obtained for this publication.
Keywords
Pacemaker lead infection; Endocarditis; HACEK; TEE
Cite this article
Wang C, Kazmi R, Shah S, Murikan M. Haemophilus parainfluenzae associated Pacemaker Lead Infection in an Active Middle-aged Man. Clin Case Rep J. 2024;5(2):1–5.
Copyright
© 2024 Chunlei Wang. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-4.0).


