Anastomosing Ovarian Hemangioma: Still A Challenge In Diagnosis – A Review Of The Morphologic And Clinical Characteristics With Differential Diagnosis
* Ermina Iljazovic;
Cickusic E;
Mustedanagic-Mujanovic J;
Karasalihovic Z;
Kuljanin M;
Sadikovic A;
Konrad–Custovic M;
Cerkez I;
Serak A;
-
* Ermina Iljazovic: Department of Pathology, Policlinic for Laboratory Diagnostic, University Clinical Center Tuzla, Bosnia and Herzegovina.
-
Cickusic E: Department of Pathology, Policlinic for Laboratory Diagnostic, University Clinical Center Tuzla, Bosnia and Herzegovina.
-
Mustedanagic-Mujanovic J: Department of Pathology, Policlinic for Laboratory Diagnostic, University Clinical Center Tuzla, Bosnia and Herzegovina.
-
Karasalihovic Z: Department of Pathology, Policlinic for Laboratory Diagnostic, University Clinical Center Tuzla, Bosnia and Herzegovina.
-
Kuljanin M: Department of Pathology, Policlinic for Laboratory Diagnostic, University Clinical Center Tuzla, Bosnia and Herzegovina.
-
Sadikovic A: Department of Pathology, Policlinic for Laboratory Diagnostic, University Clinical Center Tuzla, Bosnia and Herzegovina.
-
Konrad–Custovic M: Department of Pathology, Policlinic for Laboratory Diagnostic, University Clinical Center Tuzla, Bosnia and Herzegovina.
-
Cerkez I: Department of Pathology, Policlinic for Laboratory Diagnostic, University Clinical Center Tuzla, Bosnia and Herzegovina.
-
Serak A: Clinic for Gynecology and Obstetrics, University Clinical Center Tuzla, Bosnia and Herzegovina.
Abstract
Objectives: Anastomosing hemangioma and vascular tumors in general are relatively rare tumors that must be differentiated from their potential mimics in clinical and morphologic examinations.
Case report: In this article, we present an incidentally verified ovarian hemangioma in a slightly enlarged ovary with a well-demarcated solid and spongy tan lesion and focal hemorrhagic specks on the cut section. Microscopically, sections showed a pseudo-lobular lesion with solid nests of tightly packed cells, with clear cytoplasm and clusters of hepatoid cells with eosinophilic cytoplasm, Leydig-like cells, and were immunohistochemically positive for inhibin, calretinin, vimentin, and some for SMA. Between these cells, there were multiple, various-sized, anastomosing, thin-walled blood vessels, some filled with red blood cells. In contrast, endothelial cells were prominent, slightly pleomorphic, and positive for CD31 and CD34, confirming the vascular origin.
Conclusion: The differential diagnosis includes different benign to frank malignancies, and diagnosis requires careful and thorough study as it determines patient management. An ovarian hemangioma may be associated with stromal luteinization, which has been reported in some cases, although its pathogenesis is not clearly known.
Introduction
The basic definition of Anastomosing Hemangioma (AH) is a benign vascular tumor that can simulate angiosarcoma [1]. It was first described by Montgomery and Epstein in 2009 as a peculiar vascular lesion mimicking angiosarcoma and involving the kidneys and testes [2]. It mostly originates from the genitourinary tract, and the most common primary location is the kidneys, followed by the ovaries, colon, liver, adrenals, soft tissue, and mesentery. Stromal luteinization has been observed in ovarian hemangiomas in 11 documented cases in the English literature [3]. Despite these reports, the exact mechanism behind this association still needs to be better understood.
What Is Already Known on this Topic
Anastomosing Hemangiomas (AH): most tumors are found in the renal hilum and predominantly affect males. Histologically, AH is defined by a loosely lobular mass consisting of densely packed capillaries arranged in a sinusoidal or interconnecting pattern, resembling the red pulp of the spleen. Ovarian hemangiomas are very rare benign lesions of the ovaries. Most tumors are unilateral, less than 1.5 cm in diameter, located in the hilus or medulla of the ovary, and distinctly separated from the surrounding tissue.
Our review focuses on the full differential diagnosis and clinical presentation. We aim to increase awareness of this rare entity, facilitating the accurate diagnosis and optimal management of patients with AH.
Case Presentation
A 62-year-old postmenopausal female patient was referred to the gynecology department of University Clinical Center Tuzla; B&H with chief complaints of abdominal distension, pain, and ascites. She had two previous deliveries with a history of therapeutically controlled hypertension and diabetes type 2. Computed tomography showed a voluminous right ovary, measuring 4.5 cm x 3.5 cm x 5.5 cm, with a dominant solid component and heterogeneous intensive postcontrast imbibition, indicating an expansive process. The uterus is of an appropriate size for the age. There are no pathologically enlarged retroperitoneal or pelvic lymph nodes. A small amount of free fluid is present in the abdomen and pelvis. The left ovary demonstrated appropriate morphology for her age. Serum CA-125 levels were normal. A transabdominal hysterectomy and bilateral salpingo-oophorectomy were performed based on suspicious findings of malignancy in imaging. The specimen was submitted for histopathological examination. On gross inspection, the right ovary measured 6.0 cm x 3.5 cm x 3.0 cm and had a greyish, bumpy surface. The cut section revealed a well-defined, solid, and spongy tan lesion with areas of focal hemorrhage measuring 3.0 cm × 2.5 cm × 3.5 cm. The residual ovary showed a few small cysts with yellowish wall discoloration (Figure 1A). The left ovary had normal gross morphology, while the uterus showed several small, sharply circumscribed, firm tumors with a whorled cut surface. The endometrium was thin, with a small polypoid formation in the fundus.
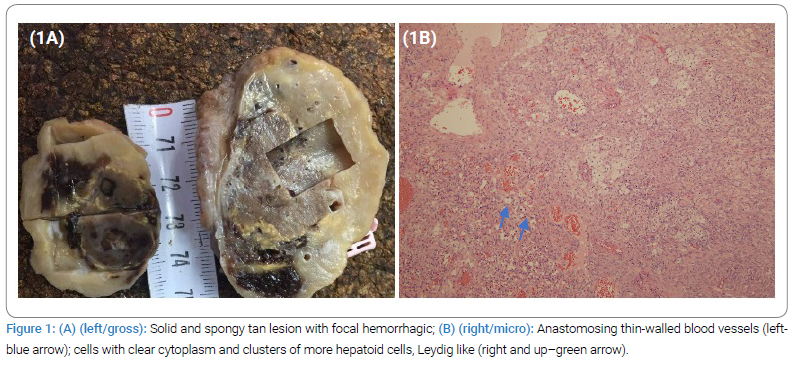
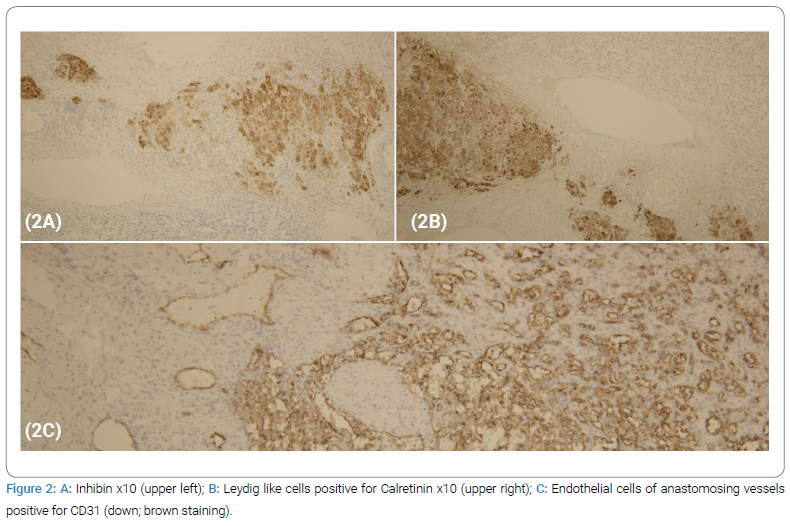
Microscopically, sections from the right ovary showed a pseudo-lobular lesion with solid nests of tightly packed cells, with clear cytoplasm, and clusters of more hepatoid cells with eosinophilic cytoplasm, Leydig-like cells (Figure 1B), and were immunohistochemically positive for inhibin, calretinin, vimentin, and some for SMA (Figure 2A). Those cells were negative for estrogen, S-100, CD99, and CD68. Additionally, microscopic examination showed a mixed cavernous and capillary hemangioma, composed of numerous thin-walled blood vessels of varying sizes filled with red blood cells. Endothelial cells were prominent, slightly pleomorphic, and positive for CD31 (Figure 2B,2C), CD34, SMA, and vimentin, confirming the vascular origin of the lesion. Mitotic activity was low, 2/10 HPF, while the proliferative index was 1%–2% (Ki-67).
Main pathology diagnosis: Unilateral ovarian haemangioma/Haemangioma ovarii unilateralis (see conclusion).
Pathohistological diagnosis also included a Glandular cystic endometrial polyp, partially infarcerated. Simple endometrial atrophy. Small uterine leiomyoma. Chronic polypoid endocervicitis. Inclusion cysts of another ovary.
Diagnosis in the Latin language: Polypus cystico-atrophicus endometrii focalis infarceratus. Atrophia simplex endometrii. Leiomyomata parva uteri. Endocervicitis chronic afocalispolypoides. Cyste inclusions ovarii lateris alteris.
Discussion
Hemangiomas are benign vascular tumors with a predilection to appear on the skin and subcutaneous soft tissue rather than in the viscera. The liver is the most common location, and hepatic hemangiomas tend to be of the cavernous type [4]. Anastamosing Hemangioma (AH) is a newly described variant of capillary hemangioma in the ovaries, usually asymptomatic, and has been reported in both children and adults [5]. These tumors were originally described as displaying a loosely lobulated architecture with areas of intravascular extension. Mostly, AH is diagnosed incidentally during an operation, autopsy, follow-up scan, or radiology procedure for some other reason.
As for symptoms, ascites are the most common sign of an abnormal finding, such as in our case. However, it may present as pseudo-Meigs syndrome, endometrial hyperplasia, or associated with elevated CA-125 levels [6]. In such cases, clinically and radiologically, suspicion of a malignant epithelial tumor may be raised. The prominent vascular network of small capillary vessels was the main component of the tumor in our case, with a pseudo-lobular pattern, nests of hepatoid cells, and a collection of cells with abundant, foamy cytoplasm. The first differential diagnosis was a primary ovarian tumor with a prominent vascular component, including a sex cord tumor/ovarian sclerosing stromal tumor and primary vascular tumor-like hemangioma or angiosarcoma. As both entities are very rare, we had to apply a wide panel of Immunohistochemistry (IHC) as well as more cuts, in order to obtain the best impression of the histological composition.
Sclerosing Stromal Tumors (SST) are extremely rare, benign ovarian neoplasms. This subtype of ovarian stromal neoplasm arises from sex cord-stromal cells and possesses unique clinical and pathological characteristics that set it apart from other stromal tumors [1]. SSTs account for 2% to 6% of ovarian stromal tumors. These tumors occur predominantly in the second and third decades of life [2], while our patient was in her seventh decade. The most common symptoms present in patients are menstrual irregularities, pelvic pain, and an abdominal mass. SSTs are typically hormonally silent. However, if they exhibit hormonal activity, they often manifest as androgenic and tend to occur predominantly during pregnancy [4]. Since its initial description by Chalvardijan and Scully in 1973, fewer than 208 cases of SSTs have been documented in the literature [5]. Due to their infrequency, the cause of SSTs remains poorly understood, and different hypotheses are under consideration. According to electron microscopy findings, one theory posits that SSTs stem from undeveloped stromal cells, while another proposes that they originate from muscle-specific actin-positive elements within the theca externa. The development of the distinctive vasculature seen in SSTs might entail the action of vascular permeability factor/vascular endothelial growth factor (VPF/VEGF) [7]. Histologically, much like anastomosing hemangiomas, they display a pseudo-lobular organization featuring densely cellular regions juxtaposed with less densely populated interlobular spaces. The cellular regions contain two types of cells: elongated fibroblastic cells and rounded to oval or polygonal cells filled with lipids. Cellular areas also show many thin-walled blood vessels, mimicking vascular tumors, but inhibin positivity suggests the diagnosis of SST. In the cellular regions, numerous delicate blood vessels resemble those seen in vascular tumors. However, the presence of inhibin positivity indicates a diagnosis of SSTs. Immunohistochemistry reveals that SSTs typically exhibit positive staining for vimentin, smooth muscle actin, alpha inhibin, calretinin, and Estrogen Receptor (ER) [8], but these markers were not detected in our case. They have a benign course and a very good prognosis with conservative surgical treatment.
Angiosarcoma was also considered in the differential diagnosis due to the hobnail-like features of the endothelial cells. The lack of cytological atypia, mitoses, low proliferative index (Ki-67), and infiltrative pattern ruled out the possibility of angiosarcoma.
Ovarian teratoma with a prominent vascular component was also one of the differential diagnoses. The diagnosis of teratoma is usually supported by the presence of skin adnexa or other ectodermal, endodermal, or mesodermal tissue components. However, our case did not contain any of these elements and, therefore, could not be classified as a teratoma.
After detailed morphological analysis, as well as the defined immunophenotype, the diagnosis of benign vascular tumor/hemangioma was established and subsequently concluded as anastomosing hemangioma. The nests of hepatoid cells, positive for inhibin and calretinin on IHC, were considered stromal luteinization, the most controversial aspect of this morphologic entity. There was no data about hormonal disorders in the history of our patient. Hemangiomas demonstrate estrogen receptors, and estrogen has a stimulative effect on the vasculature. Considering this, one hypothesis is that hyperestrogenism resulting from stromal luteinization leads to the formation of ovarian hemangiomas. In these cases, stromal luteinization is diffuse and affects both ovaries. Another hypothesis suggests that the initial event is the presence of an ovarian hemangioma, which mimics an enlarged follicle, exerting pressure on the surrounding tissue and resulting in the formation of theca-like stromal cells [9,10]. In these situations, stromal luteinization is confined to one ovary and is particularly prominent in the stroma adjacent to the tumor [11]. This could be what occurred in our case. It is well known that luteinized stromal cells produce androgens that may be converted to estrogen in the adipose tissue. This can result in both hyperandrogenism and hyperestrogenism [12]. We did not have any data about free and total testosterone in our patient because the diagnosis of anastomosing hemangioma was unexpected. However, she had no male hormonal manifestations when presented during preoperative testing. Accumulative experience indicates benign behavior, as described by Heidegger et al., who followed up on a case of renal anastomosing hemangioma in a patient for ten years [13].
Conclusion
We suggest that the differential diagnosis should include different benign to frank malignancies such as angiosarcomas, and diagnosis requires careful and thorough study and a wide panel of immunohistochemistry tests. Pathologists should be aware of anastomosing hemangioma as a rare tumor in the differential diagnosis of ovarian cystic lesions. Due to the limited number of reported cases, the choice of treatment methods and the prediction of disease outcomes remain uncertain.
What this Case Adds
Since its initial description, only a handful of cases have been documented in the ovaries. Awareness of this benign entity and correct diagnosis is essential, as it determines patient management. The etiology of ovarian hemangiomas is controversial, so it is of particular importance to present each diagnosed case with a detailed histomorphology and differential diagnosis, as we did. It is crucial for pathologists to recognize the connection with stromal luteinization, the most debated aspect of ovarian hemangioma, to prevent misdiagnosis as a steroid cell tumor with stromal vascularization or a mixed stromal vascular. The pathologist should be aware of the association with stromal luteinization, the most controversial aspect of ovarian hemangioma, to avoid misinterpretation as a steroid cell tumor with stromal vascularization or a mixed stromal-vascular tumor. In our case of the unexpected diagnosis of AH with stromal luteinization, we undertook a very detailed microscopic analysis, wide specific immunostaining, and a review of the literature in order to establish the right diagnosis with excellent prognosis and to share the experience of this still debatable entity.
Acknowledgment
We thank Professor Glenn McCluggage and Professor Xavier Matias Guiu for their support and dedicated time in defining the right terminology in the established diagnosis.
We extend our thanks to Nina Biser for her significant role in proofreading and language editing for the manuscript (Nina Z Biser; Bachelors of Arts English and Secondary Education from DePaul University, Chicago IL, USA).
Authors Contributions
Drafting the article: Iljazovic E and Karasalihovic Z; Conception and design: Cickusic E and Mustedanagic-Mujanovic J; Acquisition, analysis, and interpretation of data: Sadikovic A, Konrad–Custovic M, Kuljanin M, and Serak A; Revising it critically for important intellectual content: Iljazovic E and Cickusic E; the Approved final version of the manuscript: Iljazovic E, Karasalihovic Z, Cickusic E, and Mustedanagic-Mujanovic J.
Ethical Approval Not applicable, because this article does not contain any studies with human or animal subjects.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Informed consent was obtained for this publication.
References
- John I, Stuart LN. Anastomosing hemangioma [Internet]. Michigan (USA): pathologyoutlines.com; 2016.
- Lappa E, Drakos E. Anastomosing hemangioma: short review of a benign mimicker of angiosarcoma. Arch Pathol Lab Med. 2020;144(2):240–244.
- Gunduz M, Hurdogan O, Onder S, Yavuz E. Cystic Anastomosing Hemangioma of the Ovary: A Case Report with Immunohistochemical and Ultrastructural Analysis. Int J Surg Pathol. 2019;27(4):437–440.
- Evans J, Willyard CE, Sabih DE. Cavernous Hepatic Hemangiomas [Internet]. Treasure Island (FL): StatPearls Publishing; 2021.
- Shirazi B, Anbardar MH, Azarpira N, Robati M. An incidental ovarian mass: A case of ovarian hemangioma with prominent stromal luteinization. Medical Journal of Dr. D.Y. Patil University. 2015;8(2):227–230.
- Subbarayan D, Devaraji A, Senthilnayagam B, Ramanujam S, Nandagopalradha R. Anastomosing hemangioma of the ovary clinically masquerading as epithelial malignancy: a rare case report. J Midlife Health. 2019;10(1):48–50.
- Park CK, Kim HS. Clinicopathological Characteristics of Ovarian Sclerosing Stromal Tumor with an Emphasis on TFE3 Overexpression. Anticancer Res. 2017;37(10):5441–5447.
- Young RH. Ovarian sex cord-stromal tumors: reflections on a 40-year experience with a fascinating group of tumors, including comments on the seminal observations of Robert E. Scully, MD. Arch Pathol Lab Med. 2018;142(12):1459–1484.
- Anand MS, Shetty S, Mysorekar VV, Kumar RV. Ovarian hemangioma with stromal luteinization and HCG-producing mononucleate and multinucleate cells of uncertain histogenesis: a rare co-existence with therapeutic dilemma. Indian J Pathol Microbiol. 2012;55(4):509–512.
- Yamawaki T, Hirai Y, Takeshima N, Hasumi K. Ovarian hemangioma associated with concomitant stromal luteinization and ascites. Gynecol Oncol. 1996;61(3):438–441.
- Gücer F, Ozyilmaz F, Balkanli-Kaplan P, Mülayim N, Aydin O. Ovarian hemangioma presenting with hyperandrogenism and endometrial cancer: a case report. Gynecol Oncol. 2004;94(3):821–824.
- Huang RSP, Covinsky M, Zhang S. Bilateral ovarian capillary hemangioma with stromal luteinization and hyperandrogenism. Ann Clin Lab Sci. 2013;43(4):457–459.
- Heidegger I, Pichler R, Schäfer G, Zelger B, Zelger B, Aigner F, et al. Long-term follow up of renal anastomosing hemangioma mimicking renal angiosarcoma. Int J Urol. 2014;21(8):836–838.
Keywords
Anastomosing hemangioma; Ovary; Immunohistochemistry; Clinical characteristics
Cite this article
Iljazovic E, Cickusic E, Mustedanagic-Mujanovic J, Karasalihovic Z, Kuljanin M, Sadikovic A, et al. Anastomosing ovarian hemangioma: Still a challenge in diagnosis – a review of the morphologic and clinical characteristics with differential diagnosis. Clin Case Rep J. 2024;5(2):1–5.
Copyright
© 2024 Ermina Iljazovic. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-4.0).


