Hypersensitivity to Acetylsalicylic Acid or Primary Cutaneous Th2 Cell Lymphoma? Cognitive Autopsy
* Werber-Bandeira L;
Bandeira IM;
Dias dos Santos AM;
Marcos Manuel NA;
Mitre PPS;
Rebello CM;
Sabrá S;
Sabrá-Filho A;
Braga BB;
Sabrá A;
Bandeira TL;
-
* Werber-Bandeira L: Clinical and Experimental Immunology and Allergy Service, Santa Casa da Misericórdia do Rio de Janeiro/Imunoderm Clinic, Rio de Janeiro, RJ, Brazil; Estácio de Sa University/IDOMED. Rio de Janeiro, RJ, Brazil.
-
Bandeira IM: Clinical and Experimental Immunology and Allergy Service, Santa Casa da Misericórdia do Rio de Janeiro/Imunoderm Clinic, Rio de Janeiro, RJ, Brazil.
-
Dias dos Santos AM: Clinical and Experimental Immunology and Allergy Service, Santa Casa da Misericórdia do Rio de Janeiro/Imunoderm Clinic, Rio de Janeiro, RJ, Brazil.
-
Marcos Manuel NA: Clinical and Experimental Immunology and Allergy Service, Santa Casa da Misericórdia do Rio de Janeiro/Imunoderm Clinic, Rio de Janeiro, RJ, Brazil.
-
Mitre PPS: Clinical and Experimental Immunology and Allergy Service, Santa Casa da Misericórdia do Rio de Janeiro/Imunoderm Clinic, Rio de Janeiro, RJ, Brazil.
-
Rebello CM: Clinical and Experimental Immunology and Allergy Service, Santa Casa da Misericórdia do Rio de Janeiro/Imunoderm Clinic, Rio de Janeiro, RJ, Brazil.
-
Sabrá S: University of the Grand Rio, Duque de Caxias, Rio de Janeiro, RJ, Brazil; Pediatric Endoscopy Service, Antonio Pedro University Hospital, Niterói-Rio de Janeiro, RJ, Brazil.
-
Sabrá-Filho A: University of the Grand Rio, Duque de Caxias, Rio de Janeiro, RJ, Brazil.
-
Braga BB: University of the Grand Rio, Duque de Caxias, Rio de Janeiro, RJ, Brazil.
-
Sabrá A: Food Allergy and Autism Unit of the Clinical Immunology Service, Santa Casa da Misericórdia do Rio de Janeiro, Rio de Janeiro, RJ, Brazil.
-
Bandeira TL: Clinical and Experimental Immunology and Allergy Service, Santa Casa da Misericórdia do Rio de Janeiro/Imunoderm Clinic, Rio de Janeiro, RJ, Brazil.
Abstract
A 68-year-old male presented with persistent pruritic erythroderma following an acute myocardial infarction, initially suspected to be hypersensitivity to Acetylsalicylic Acid (ASA). Despite the cessation of ASA, the condition persisted, leading to further investigation. Physical examination revealed erythroderma, nail dystrophy, livedo reticularis, and infiltrated hyperchromic lesions. Diagnostic considerations included ASA hypersensitivity, Mycosis Fungoides (MF), and psoriasis. Histopathological examination and immunohistochemical analysis identified perivascular and interstitial lymphocytic infiltrates with CD3, CD4, CD5, CD20, CD45RA, and CD45RO positivity, consistent with MF, a subtype of primary Cutaneous T-Cell Lymphoma (CTCL). The diagnosis was confirmed for primary cutaneous CD4 T-cell lymphoma in the erythrodermic phase, and the patient was treated with Photochemotherapy (PUVA).
This case underscores the diagnostic challenges associated with CTCL, often misinterpreted as benign conditions such as drug hypersensitivity. Detailed patient history, physical examination, and comprehensive diagnostic protocols, including immunophenotyping, are essential for accurate diagnosis. The report also highlights the concept of “cognitive autopsy,” reflecting on the cognitive processes leading to a change from an initial misdiagnosis of ASA hypersensitivity to the accurate identification of CTCL. The case exemplifies the importance of integrating clinical judgment with detailed diagnostic workups to avoid diagnostic errors and ensure appropriate patient management.
Introduction
The CTCL, including MF, represents a group of skin malignancies arising from T-lymphocytes, presenting with diverse cutaneous manifestations. Diagnosis is often challenging due to its clinical resemblance to more benign conditions such as drug hypersensitivity reactions. This case report highlights the diagnostic journey of a 68-year-old male initially suspected of ASA hypersensitivity, underscoring the importance of comprehensive diagnostic protocols and the role of immunophenotyping in distinguishing CTCL from other dermatological conditions.
Case Presentation
Man, 68-year-old, businessman, married and born in Rio de Janeiro, RJ, referred for investigation of “aspirin allergy.”
History of Present Illness (HPI): Sick for one year. After a coronary event of Acute Myocardial Infarction (AMI), during hospitalization, he was medicated with ASA and antihypertensives, starting an itchy skin condition that persists to this day. Suspension of medications for a period of 30 days showed no improvement. He was referred by the cardiologist for research into “aspirin allergy.”
Medications in use: losartan, atorvastatin, Acetylsalicylic Acid (AAS), and cinnarizine.
Upon physical examination it was observed: erythroderma (Figure 1) with a positive glass slide sign; Nail dystrophy in all 10 fingers (Figure 2); Livedo reticularis (Figure 3) and Infiltrated hyperchromic lesion (Figure 4) on the dorsal region.
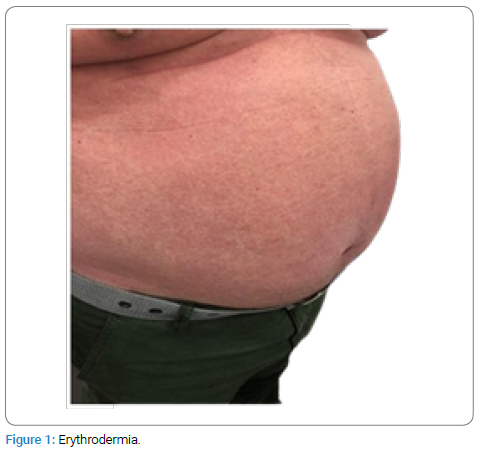
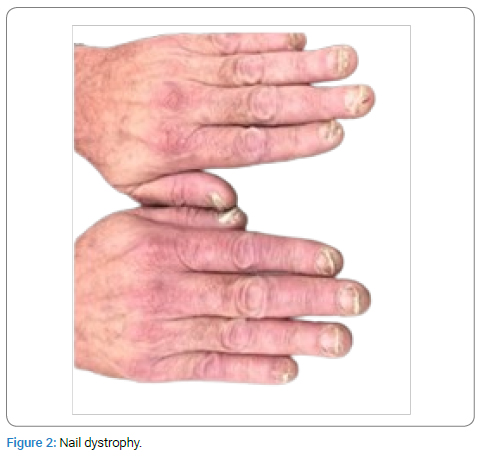
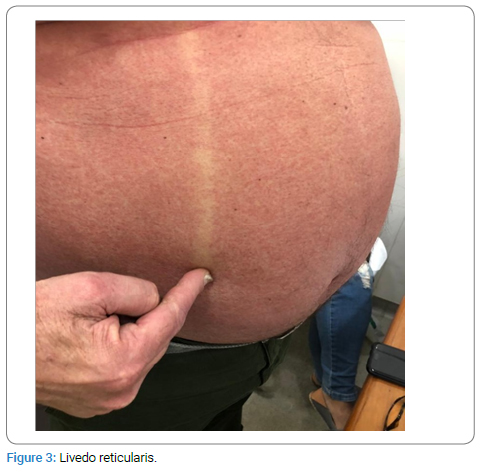
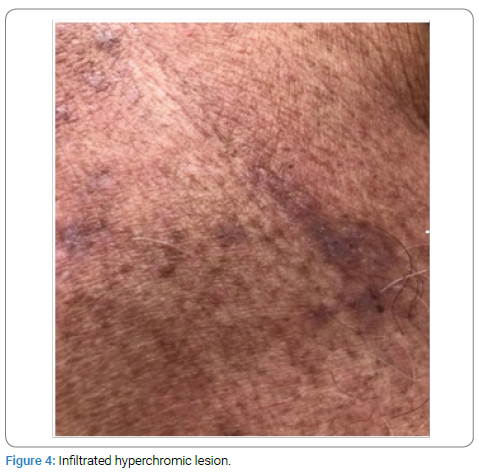
Diagnostic Hypothesis: 1) Hypersensitivity to ASA? 2) Mycosis Fungoides? 3) Psoriasis?
Requested exams: Blood tests, lymphocyte subsets, and biopsy of the dorsal lesion for routine histology.
Results
PCR: 1,38
CD8: 31,8% (13,8 a 27,4) 534 mm3(330 a 1008 mm3).
CD4: 39% (31 a 56%) 659 mm3 (507 a 1496).
CD3: 81,2 (55,5 a 75,2) 1681 (1350 a 2750).
CD56: 6,3 (7 a 22)
CD16: 125 (137 a 567).
Histology: Serial sections show dermis with dense infiltration of lymphocytes forming perivascular cuffs, interposing in the interstitium of collagen bundles and blurring the epidermis. The findings are consistent with a Perivascular and Interstitial Mycosis Fungoides.
Second request: Immunohistochemistry and Blood test: Sézary cells.
Results: Diffuse positivity for CD3/CD4/CD5/CD20/CD45RA/CD45RO in perivascular lymphocytes and lymphocytes aligned to the basal layer. CD7: Positive in 40% of CD3-labeled cells, CD8: Multifocal positivity in perivascular lymphocytes, negative in basal layer-aligned lymphocytes. CD30: Sparse cells permeate the infiltrate. Ki-67, p53: positive in about 10% of cells. The search for Sézari Cells was negative.
The diagnostic conclusion was: primary cutaneous CD4 T-cell lymphoma, erythrodermic phase with characteristic nail lesions. The alterations of the lymphocyte subpopulations and epidermal mononuclear infiltrate define the diagnosis.
The therapeutic approach adopted was photochemotherapy. PUVA (psoralen Ultra Violet A) [1].
Discussion
Primary cutaneous T-cell lymphoma is a type of skin cancer that originates from T lymphocytes, causing various cutaneous manifestations and lymphocyte epidermotropism. The patch/plaques and erythrodermic phase indicate a condition in which much or all of the skin on the patient’s body is inflamed, which is a serious feature that can occur in cutaneous T-cell lymphomas. In this T1 stage patient, skin lesions can be small spots (flat lesions), papules (small lumps), and/or plaques (elevated or lowered flat lesions), but the lesions cover less than 10% of the skin surface [2].
MF is the most common subtype of cutaneous T-cell lymphoma. Early-stage disease is characterized by superficial infiltrates of small—to medium-sized monoclonal atypical epidermotropic T lymphocytes.
Diagnosis of early cutaneous lymphomas, including CTCL, is often challenging due to the similarity of cutaneous symptoms to more common and less severe conditions, such as allergic reactions, in the erythrodermic phase. This may lead to an initial suspicion of an allergy, such as aspirin allergy, based on skin manifestations. However, to establish the diagnosis of cutaneous lymphoma, detailed analyses of HPI, physical examination of the skin and cells that infiltrate the epidermis, epidermal mononuclear infiltrates, and observation of changes in lymphocyte subpopulations are necessary [3,4].
In this context, CD7 and CD30 were evaluated. CD7 is the first CD3 cell marker to lose expression in the progression of CTCL, while CD30 is a cell surface receptor expressed to varying degrees in many lymphomas, including cutaneous anaplastic large cell lymphoma (C-ALCL).
Staining for CD30 is frequently used in cases of T-cell proliferation in the epidermis and/or dermis. Typically, the CD30-negative group of T-cell lymphomas includes classic mycosis fungoides and variants of CTCL that present an increased CD4:CD8 ratio. Cluster differentiation CD30+ has been identified as an important therapeutic target in lymphomas [5].
The T-cell infiltrate in the lower third of the dermis and subcutaneous tissue can be stained for CD56, which is a marker found in Natural Killer (NK) cells, a subset of CD3-negative T-cells, and in panniculitis-like T-cell lymphomas.
Ki-67 is a protein that represents a proliferation marker for human tumor cells. Positivity for Ki-67, p53 in about 10% of cells, as demonstrated in patients, suggests expansive cell proliferation [6]. The molecular mechanisms by which advanced cases of CTCL (MF/Sezary syndrome) undergo Large Cell Transformation (LCT) and develop the morphologic appearance of a large cell lymphoma are undefined, but evaluating p53 expression in correlation with other markers and clinical parameters to get a comprehensive view of its role in disease progression was fundamental. The p53 positivity in about 10% of cells is a significant finding that could have implications for the diagnosis [7].
The loss of pan T-cell markers, such as CD2, CD5, and CD7, in CD4+ T cells within lesional skin supports the diagnosis of MF. Notably, the absence of CD2 and CD5 is rarely observed in early MF. Expression of CD2 or CD5 in less than 50% of the infiltrating T cells is highly specific but has only about 10% sensitivity for MF. In the early stages of the disease, where the number of tumor cells in the dermis is limited, the absence of these T-cell markers may be detected solely in the epidermis in some cases.
CD20-positive primary cutaneous T-cell lymphoma is rare. One possible explanation for the presence of CD20+ T cells is that these lymphomas may originate from the subset of normal T cells that express CD20, or CD20 may serve as an activation marker [8]. The CD45 tyrosine phosphatase isoforms have different molecular weights and are differentially expressed in hematopoietic cells. CD45RA T cells are “naive” cells that do not respond to antigen recall and are prominent in cord blood. In contrast, CD45R O T cells are “memory” T cells that proliferate in response to recall antigens. Thus, we can say that CD45RO T cells are committed memory cells [9].
Final Considerations
The term “cognitive autopsy” is increasingly used in medicine, particularly in diagnostic discussions, because it encapsulates the idea of retrospectively analyzing the cognitive processes involved in medical decision-making. In medical practice, diagnoses are often complex and multifaceted, requiring clinicians to integrate a wide range of information, including patient history, symptoms, test results, and their own clinical judgment. However, diagnostic errors still occur, and besides heart disease and cancer, medical error is actually one of the leading causes of death.
Among the various medical errors that can result in injury and death, diagnostic failure is considered the most significant. The cognitive autopsy in medicine involves examining these errors by dissecting the cognitive processes that led to them. By conducting cognitive autopsies, medical professionals can identify cognitive biases, heuristics, knowledge gaps, and other factors that may have contributed to diagnostic errors. This approach allows for a deeper understanding of the underlying cognitive mechanisms at play in medical decision-making and provides opportunities for improving diagnostic accuracy and patient care.
We report here a case of MF in a 68-year-old male initially diagnosed with an ASA allergy. In this case, an aspirin allergy was suspected due to skin manifestations. However, detailed analyses of the physical examination, including the cells infiltrating the skin (epidermal mononuclear infiltrates) and lymphocyte changes, suggested a CTCL diagnosis.
This report clearly illustrates the importance of anamnesis and detailed physical examination, the use of a protocol for differential diagnosis, and the application of immunophenotyping of cutaneous lymphoma as standard practices for achieving an accurate diagnosis and avoiding incorrect treatment that could harm the patient. Furthermore, this was a typical case in which conducting a cognitive autopsy led to reaching the final diagnosis.
The diagnosis of MF is challenging and often delayed, partly due to the heterogeneity and location of the lesions, which can mimic benign inflammatory dermatoses. Clinicians need to be aware of and have a general understanding of how immunophenotyping is used to characterize lymphocytic proliferation and correlate it with clinical findings.
Conflict of Interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Informed consent was obtained for this publication.
References
- Werber-Bandeira L, Herdy AM, Pagani EA, Filgueira AL. Primary cutaneous T cell lymphomas: photochemotherapy immunomodulation with analysis of the inflammatory-expansive cellular dynamic. Dermatol Ther. 2014;27(2):74–78.
- Cutaneous Lymphoma Foundation. A Patient’s Guide to Understanding Cutaneous Lymphoma [Internet]. Michigan: CLFoundation; 2024.
- Gallardo F, Pujol RM. Diagnóstico y tratamiento de los linfomas cutáneos primarios de células B. Actas Dermo-Sifiliográficas. 2004;95(9):537–547.
- Moreno-Ramírez D, Saval AH, Martínez FC. Diagnóstico y tratamiento de los linfomas cutáneos primarios de células T. Medicina Cutánea Ibero-Latino-Americana. 2003;31(2):75–100.
- Miyagaki T. Diagnosis of Early Mycosis Fungoides. Diagnostics. 2021;11(9):1721.
- Strutton J. Cutaneous infiltrates: lymphomatous and leukemic. In: Weedon D, editor. Skin Pathology. 3rd ed. Philadelphia, Pa: Churchhill Livingston; 2010;971–1005.
- Li G, Chooback L, Wolfe JT, Rook AH, Felix CA, Lessin SR, et al. Overexpression of p53 protein in cutaneous t cell lymphoma: relationship to large cell transformation and disease progression. J Invest Dermatol. 1998;110(5):767–770.
- Martin B, Stefanato C, Whittaker S, Robson A. Primary cutaneous CD20-positive T-cell lymphoma. J Cutan Pathol. 2011;38(8):663–669.
- LaSalle JM, Hafler DA. The coexpression of CD45RA and CD45RO isoforms on T cells during the S/G2/M stages of cell cycle. Cell Immunol. 1991;138(1):197–206.
Keywords
Primary cutaneous t-cell lymphoma; Acetylsalicylic acid hypersensitivity; Cognitive autopsy; Clinical diagnosis
Cite this article
Werber-Bandeira L, Bandeira IM, Dias dos Santos AM, Marcos Manuel NA, Mitre PPS, Rebello CM, et al. Hypersensitivity to acetylsalicylic acid or primary cutaneous Th2 cell lymphoma? Cognitive autopsy. Clin Case Rep J. 2024;5(3):1–4.
Copyright
© 2024 Werber-Bandeira L. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-4.0).




