Space-Occupying Lesion in the Pancreas – A Diagnostic Challenge
* Eszter Daru;
Groth S;
* Kleber M;
-
* Eszter Daru: Institute for General Internal Medicine, Klinik Hirslanden, Zurich, Switzerland.
-
Groth S: Gastroenterology Center, Klinik Hirslanden, Zurich, Switzerland.
-
* Kleber M: Institute for General Internal Medicine, Klinik Hirslanden, Zurich, Switzerland.
-
May 30, 2025 |
-
Volume: 6 |
-
Issue: 2 |
-
Views: 2400 |
-
Downloads: 901 |
Abstract
A 79-year-old patient presented with the following symptoms: abdominal pain radiating to the back, subfebrile temperatures, loss of appetite, and night sweats. Imaging diagnostics suggested a pancreatic carcinoma, but histological confirmation through EUS-FNA was not possible. The patient declined the possibility of resection. After four weeks, the suspicion of a solid space-occupying lesion could not be confirmed. This case highlights the diagnostic challenges in evaluating ambiguous pancreatic masses, particularly when a malignant process is suspected but cannot be conclusively confirmed through imaging or histopathology.
Abbreviations
AIP: Autoimmunpancreatitis
CA 19-9: Carbohydrate Antigen 19-9
CEA: Carcino Embryonic Antigen
CRP: C-Reactive-protein
CT: Computer Tomographie
EUS-FNA: Endoscopic Ultrasound-Guided Fine-Needle Aspiration
MRCP: Magnetic Resonance Cholangiopancreatography
PSC (Classification): Papanicolaou Society of Cytopathology
Background
If there is suspicion of a solid space-occupying lesion in the pancreas, early detection of possible neoplastic lesions should be undertaken. An expanded diagnostic algorithm, including both invasive and non-invasive measures, is available for this purpose. Precise imaging techniques, particularly in cases of unclear pancreatic space-occupying lesions, are crucial for enabling an accurate diagnosis, and these techniques significantly contribute to avoiding overtreatment [18]. The differentiated use of imaging techniques such as CT and MRI is also critical for distinguishing pancreatic carcinomas from other pancreatic pathologies, which is particularly important in this case [20]. If the examination results lead to a diagnosis of pancreatic carcinoma, it is often already at an advanced stage, leading to a poor prognosis [1]. However, rare cases of spontaneous regression can provide important insights into natural disease dynamics and potential immunological mechanisms. These insights may be considered in future therapeutic strategies and offer a deeper understanding of the complexity of cancer diseases [23].
Case Presentation
A 79-year-old female patient presented to the emergency department with diffuse abdominal pain radiating to the back, associated with sub febrile temperatures over the previous week. Additionally, she reported loss of appetite and night sweats. On physical examination, the patient was cardiopulmonary stable and presented with a sub febrile temperature of 37.2°C/98.96 F. Tenderness was noted in the lower and upper abdomen without signs of peritonitis, and normal bowel sounds were present. Laboratory tests revealed leukocytosis of 11.6 G/l (normal range 3.60 G/l–10.50 G/l) and an elevated CRP level of 89 mg/l (normal < 5.0 mg/l). Lipase levels were within the normal range. An abdominal CT scan was performed, showing a poorly defined hypodense lesion of 2.6 cm x 3.4 cm in the body of the pancreas, which is highly suspicious for pancreatic carcinoma (Figure 1).
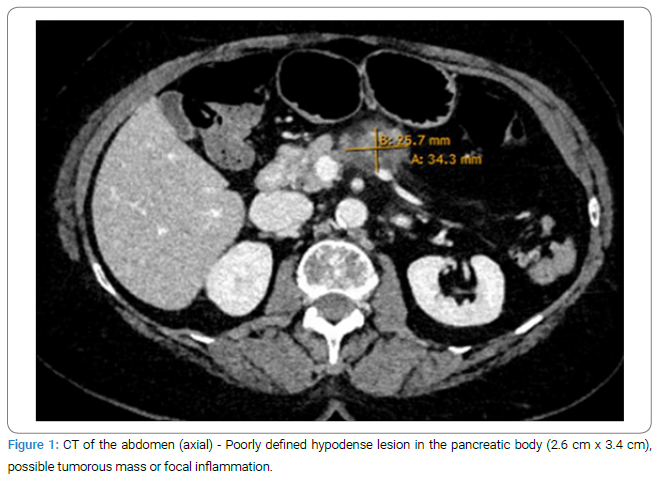
Further diagnostic steps: A histopathological analysis was planned to further investigate the suspected diagnosis, and a sample was obtained through an EUS-FNA. Cytology revealed a PSC III classification, so no definitive diagnosis could be made. A repeated puncture four days later also showed no evidence of carcinoma. Additional diagnostic testing with the tumor markers CA 19-9 and CA 125 yielded no pathological findings. As part of the tumor staging, thoracic distant metastases were excluded by CT of the chest. The MRCP still showed a strong suspicion of a soft tissue neoplasm. Incidentally, stool culture revealed a confirmed Clostridium difficile infection, which was treated with antibiotics. The further course was discussed in the internal tumor board, where surgical resection was recommended. However, the patient clearly declined the surgery. Given the acute infection, it was agreed that clinical and CT follow-up of the mass would be conducted after the Clostridium colitis had resolved, which was possible after four weeks. Surprisingly, the previously defined pancreatic body mass could no longer be visualized (Figure 2).
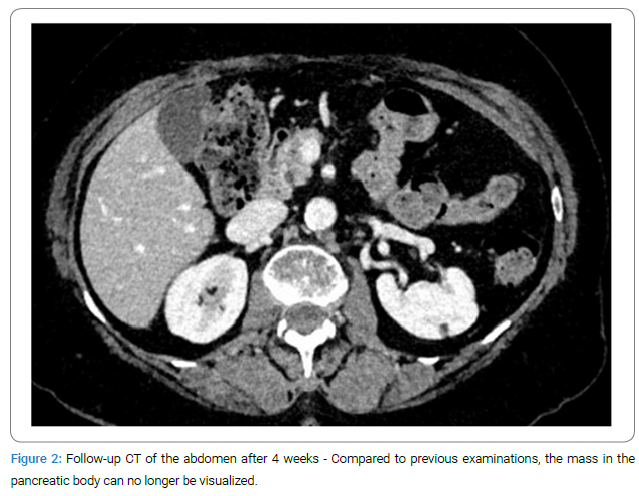
Given the results from imaging and histopathological analysis, the question arises as to whether the initially diagnosed pancreatic lesion was a focal inflammation or a pancreatic carcinoma [22]. There is a diagnostic challenge in distinguishing mass-forming pancreatic lesions. Secondary imaging features, such as the infiltration of the pancreatic duct by the lesion, the “capsule-like rim sign,” and the pancreatic duct-to-parenchymal ratio, are crucial in differentiating between an inflammatory mass and carcinoma [24]. These considerations are particularly relevant when, as in our case, the histopathology does not provide a definitive diagnosis.
Discussion
Based on the patient’s history and the imaging findings, pancreatic carcinoma was the primary concern. A stepwise diagnostic approach is used for the etiological investigation of a pancreatic mass [2–4]. Screening of asymptomatic individuals is not recommended according to current evidence-based guidelines [5]. When a malignant pancreatic mass is suspected in the initial diagnostic workup (clinical evaluation, laboratory tests, abdominal ultrasound), as in our case, a contrast-enhanced abdominal CT is indicated. This allows for the detection of the extent of the lesion and potential abdominal metastases, as well as the assessment of resectability [7]. To improve diagnostic accuracy alongside the lipase and amylase levels, as well as tumor markers such as CA 19-9, CA 125, and CEA (a marker initially used for colorectal carcinomas) should also be measured [5,8,17]. A hypodense lesion in the pancreatic body was observed in the abdominal CT scan of our patient. Both a tumor mass and focal inflammation were considered. There were no laboratory signs suggestive of acute pancreatitis, and morphologically, the pancreas appeared rather atrophic in the CT image [6]. The patient had no history of acute pancreatitis. The importance of recognizing typical and atypical imaging features for the differential diagnostic evaluation of pancreatic masses is emphasized, as this can be crucial for accurately distinguishing between neoplastic and non-neoplastic lesions [21]. The necessity for a differentiated approach in the imaging of pancreatitis, including both acute and chronic forms, is also highlighted, particularly when distinguishing pancreatic carcinomas from non-inflammatory conditions [20]. These findings are particularly valuable when assessing imaging results in unclear cases, such as the one presented, to enable precise differentiation between different types of pancreatic lesions. The suspected diagnosis of pancreatic carcinoma in our case was investigated through endosonography (EUS). Research-based on a rare case illustrates how complex pathological processes can lead to misinterpretations in diagnostics, underscoring the need for comprehensive analysis in similar cases [10]. It is noted that in rare cases, spontaneous regression of a pancreatic lesion could be associated with an underlying malignant condition, which could open new perspectives in the differential diagnostic evaluation of pancreatic lesions, especially in patients with elevated IgG4 levels and atypical EUS findings that may indicate autoimmune pancreatitis (AIP) [28]. Furthermore, EUS, especially through novel techniques like contrast-enhanced EUS and EUS-guided needle-based confocal laser endomicroscopy, has established itself as an essential tool in diagnosing pancreatic cystic lesions. These methods improve the differentiation between malignant and benign lesions and reduce the need for invasive procedures by providing more precise diagnoses [19]. Distinguishing the four types of pancreatic and peripancreatic collections based on the presence or absence of necrosis and the time elapsed since the onset of acute pancreatitis is crucial for accurate diagnosis [18].
Endosonography can also be used for biopsy sampling via fine-needle aspiration for differential diagnosis. In the repeated cytopathological examinations of our patient, explanations such as pancreatic lipoma, cystic lesion or neoplasia, intraductal papillary-mucinous neoplasia, and distant metastases were ruled out [12,13]. Possible differential diagnoses should be excluded or not confirmed. To increase the sensitivity and specificity of the diagnostics and for pretherapeutic staging purposes, an abdominal MRI, MRCP [9], a chest CT for preoperative staging, a PET-CT and an optional staging laparoscopy [5] are recommended. The staging laparoscopy can alter the results of imaging procedures regardless of their quality [11]. A surgical R0 resection can offer a good long-term survival rate [14], and the patient’s age alone should not be an exclusion criterion for surgical interventions [15]. The observation of the spontaneous disappearance of a pancreatic lesion in our case leads to important considerations regarding potential causes. While the literature documents spontaneous regressions in various malignant conditions, in our case, the definitive diagnosis of malignancy remains absent. Imaging and clinical progression rather suggest a non-malignant etiology [16]. The potential role of autoimmune pancreatitis (AIP), especially Type 1 AIP characterized by a response to steroid therapy, is emphasized, although specific IgG4 serum levels were not determined [29]. The mention of histological findings of pronounced granulocytic acute inflammation and degenerated acinar cells could suggest AIP even without direct evidence of IgG4 or steroid therapy use [25]. Furthermore, the study underscores the role of endoscopic ultrasound (EUS) in unclear bile duct dilatations, particularly after inconclusive MRCP results, highlighting the diagnostic precision of this procedure [25], which emphasizes the need for careful selection of diagnostic tools. Acute and chronic pancreatitis are significant risk factors for pancreatic carcinoma, and differentiation can be challenging due to similar symptoms. Imaging techniques such as EUS and CT supplemented by targeted biopsies are crucial for differentiating between these two conditions [26]. Research on the challenge of distinguishing AIP from pancreatic carcinomas reveals that AIP is often mistaken for pancreatic carcinoma, which can lead to unnecessary pancreatectomies. The study emphasizes the role of EUS-FNA in differential diagnosis and the effectiveness of corticosteroids in treatment [27]. This highlights the importance of accurate diagnostic evaluation in unclear pancreatic lesions to avoid misdiagnoses and unnecessary surgical interventions.
Conclusion
This case of an unclear pancreatic mass highlights the diagnostic challenges and importance of thorough evaluation when there is suspicion of pancreatic carcinoma. A small number of pancreatic carcinomas are discovered at an early stage, which underscores the need for effective early detection. The observation of a temporary pancreatic lesion in our case, which was resolved without surgical intervention, emphasizes the importance of precise initial diagnostics. Monitoring and diagnosing AIP are important measures, though they were not relevant in this particular case. Advanced imaging techniques are crucial for distinguishing between malignant and non-malignant lesions and contribute to optimizing patient care. The rare observation of spontaneous regression of a pancreatic lesion highlights the complexity of tumor dynamics and the importance of a comprehensive, individualized diagnostic and therapeutic approach. These findings emphasize the need to consider all possible diagnoses in the diagnostic assessment of pancreatic masses and the necessity of continuous clinical evaluation to avoid overtreatment and ensure the best possible care.
Leading Points
- Differential diagnosis in pancreatic lesions: This case highlights the importance of accurate differential diagnosis in unclear pancreatic lesions, particularly when malignancy is suspected.
- Importance of comprehensive diagnostics: The case emphasizes the need for an extensive diagnostic evaluation, including imaging and histopathology.
- Adherence to evidence-based guidelines: The case underscores the importance of following evidence-based guidelines in the diagnostic process, especially with typical findings.
- Autoimmune processes in pancreatic pathology: The case sheds light on the role of autoimmune processes, particularly autoimmune pancreatitis, in pancreatic pathology.
Conflict of Interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Informed consent was obtained for this publication.
References
- Yang J, Xu R, Wang C, Qiu J, Ren B, You L. Early screening and diagnosis strategies of pancreatic cancer: a comprehensive review. Cancer Commun (Lond). 2021;41(12):1257–1274.
- Ducreux M, Cuhna ASa, Caramella C, Hollebecque A, Burtin P, Goéré D, et al. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015:26 Suppl 5:v56–v68.
- Huang J, Lok V, Ngai CH, Zhang L, Yuan J, Lao XQ, et al. Worldwide Burden of, Risk Factors for, and Trends in Pancreatic Cancer. Gastroenterology. 2021;160(3):744–754.
- Tempero MA, Malafa MP, Al-Hawary M, Behrman SW, Benson AB, Cardin DB, et al. Pancreatic adenocarcinoma, Version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19(4):439–457.
- Seufferlein T, Mayerle J, Böck S, Brunner T, Ettrich TJ, Grenacher L, et al. S3-Leitlinie zum exokrinen Pankreaskarzinom – Kurzversion 2.0 – Dezember 2021, AWMF-Registernummer: 032/010OL. Z Gastroenterol. 2022;60(6):991–1037.
- Bekkali NLH, Oppong KW. Pancreatic ductal adenocarcinoma epidemiology and risk assessment: Could we prevent? Possibility for an early diagnosis. Endosc Ultrasound. 2017;6(Suppl 3):S58–S61.
- Puckett Y, Garfield K. Pancreatic Cancer [Internet]. New York City: Stat Pearls; 2023.
- Hanna-Sawires RG, Schiphuis JH, Wuhrer M, Vasen HFA, van Leerdam ME, Bonsing BA, et al. Clinical perspective on proteomic and glycomic biomarkers for diagnosis, prognosis, and prediction of pancreatic cancer. Int J Mol Sci. 2021;22(5):2655.
- Wu H, Ou S, Zhang H, Huang R, Yu S, Zhao M, et al. Advances in biomarkers and techniques for pancreatic cancer diagnosis. Cancer Cell Int. 2022;22(1):220.
- Iglesias-Garcia J, de la Iglesia-Garcia D, Olmos-Martinez JM, Lariño-Noia J, Dominguez-Muñoz JE. Differential diagnosis of solid pancreatic masses. Minerva Gastroenterol Dietol. 2020;66(1):70–81.
- Doucas H, Sutton CD, Zimmerman A, Dennison AR, Berry DP. Assessment of pancreatic malignancy with laparoscopy and intraoperative ultrasound. Surg Endosc. 2007;21(7):1147–1152.
- Liu KL, Wu T, Chen PT, Tsai YM, Roth H, Wu MS, et al. Deep learning to distinguish pancreatic cancer tissue from non-cancerous pancreatic tissue: a retrospective study with cross-racial external validation. Lancet Digit Health. 2020;2(6):e303–e313.
- Laoveeravat P, Abhyankar PR, Brenner AR, Gabr MM, Habr FG, Atsawarungruangkit A. Artificial intelligence for pancreatic cancer detection: Recent development and future direction . Artif Intell Gastroenterol.2021; 2(2):56–68.
- Doi R, Imamura M, Hosotani R, Imaizumi T, Hatori T, Takasaki K, et al. Japan Pancreatic Cancer Study Group. Surgery versus radiochemotherapy for resectable locally invasive pancreatic cancer: final results of a randomized multi-institutional trial. Surg Today. 2008;38(11):1021-1028.
- Ansari D, Aronsson L, Fredriksson J, Andersson B, Andersson R. Safety of pancreatic resection in the elderly: a retrospective analysis of 556 patients. Ann Gastroenterol. 2016;29(2):221–225.
- Midha S, Chawla S, Garg PK. Modifiable and non-modifiable risk factors for pancreatic cancer: A review. Cancer Lett. 2016;381(1):269–277.
- Cohen JD, Javed AA, Thoburn C, Wong F, Tie J, Gibbs P, et al. Combined circulating tumor DNA and protein biomarker-based liquid biopsy for the earlier detection of pancreatic cancers. Proc Natl Acad Sci U S A. 2017;114(38):10202–10207.
- Case BM, Jensen KK, Bakis G, Enestvedt BK, Shaaban AM, Foster BR. Endoscopic interventions in acute pancreatitis: what the advanced endoscopist wants to know. Radiographics. 2018;38(7):2002–2018.
- Alvarez-Sánchez MV, Napoléon B. New horizons in the endoscopic ultrasonography-based diagnosis of pancreatic cystic lesions. World J Gastroenterol. 2018;24(26):2853–2866.
- Morana G, Beleù A, Nistri F, Venturini S. Imaging of Pancreatitis. In: Massani M, Stecca T, editors. Multidisciplinary Management of Acute and Chronic Pancreatitis. Intech Open. 2023.
- Ozaki K, Ikeno H, Kaizaki Y, Maeda K, Higuchi S, Kosaka N, et al. Pearls and pitfalls of imaging features of pancreatic cystic lesions: a case-based approach with imaging–pathologic correlation. Jpn J Radiol. 2021;39:118–142.
- Naffouje SA, Salti GI. Transient exocrine pancreatic insufficiency: first report of an unrecognized complication of cytoreductive surgery and HIPEC. Anticancer Res. 2018;38(4):2353–2358.
- Minacapelli CD, Leuszkiewicz P, Patel A, Catalano C, Abdelsayed G, Lalos A, et al. The spontaneous regression of primary gastrointestinal malignancies: an observational review. Cureus. 2022;14(12):e32970.
- Wolske KM, Ponnatapura J, Kolokythas O, Burke LMB, Tappouni R, Lalwani N. Chronic pancreatitis or pancreatic tumor? A problem-solving approach. Radiographics. 2019;39(7):1965–1982.
- Atalla H, Menessy A, Hakim H, Shiomi H, Kodama Y, Ghoneem E. Clinical utility of linear endosonography in patients with unexplained biliary dilatation and negative MRCP, with predictors for detection of neoplastic lesions. Egypt Liver J. 2022;12:1–10.
- Rho S, Martin S, Nigogosyan Z, Kushnir V, Mintz AJ, Hu ZI. Pancreatic tail cancer in the setting of pancreatitis with a review of the literature: A case report. Clin Case Rep. 2023;11(10):e8023.
- Hsu WL, Chang SM, Wu PY, Chang CC. Localized autoimmune pancreatitis mimicking pancreatic cancer: Case report and literature review. J Int Med Res. 2018;46(4):1657–1665.
- Ohtsubo K, Yamashita K, Yanagimura N, Suzuki C, Tanimoto A, Nishiyama A, et al. Multiple malignant lymphomas of the bile duct developing after spontaneous regression of an autoimmune pancreatitis-like mass. Intern Med. 2021;60(3):409–415.
- Omiyale AO. Autoimmune pancreatitis. Gland Surg. 2016;5(3):318–326.
Cite this article
Daru E, Groth S, Kleber M. Space-occupying lesion in the pancreas – a diagnostic challenge. Clin Case Rep J. 2025;6(2):1–5.
Copyright
© 2025 Eszter Daru. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-4.0).


