Mastectomy for Severe Radiation-Induced Fibrosis Following Breast Conservation Therapy
* Greenblat M;
* Johnson C;
Mangino A;
Skoracki R;
Abdulwaasey M;
Tozbikian G;
Sorkin M;
Taylor C;
* William E. Carson;
-
* Greenblat M: Department of Surgery, The Ohio State University Wexner Medical Center, Columbus, OH, USA.
-
* Johnson C: The Arthur G. James Comprehensive Cancer Center and Richard J. Solove Research Institute, The Ohio State University Wexner Medical Center, OH, USA.
-
Mangino A: Department of Surgery, The Ohio State University Wexner Medical Center, Columbus, OH, USA.
-
Skoracki R: Department of Plastic Surgery, The Ohio State University Wexner Medical Center, OH, USA.
-
Abdulwaasey M: Department of Pathology, The Ohio State University Wexner Medical Center, OH, USA.
-
Tozbikian G: Department of Pathology, The Ohio State University Wexner Medical Center, OH, USA.
-
Sorkin M: Department of Plastic Surgery, The Ohio State University Wexner Medical Center, OH, USA.
-
Taylor C: Department of Radiology, The Ohio State University Wexner Medical Center, OH, USA.
-
* William E. Carson: Department of Surgery, The Ohio State University Wexner Medical Center, Columbus, OH, USA; The Arthur G. James Comprehensive Cancer Center and Richard J. Solove Research Institute, The Ohio State University Wexner Medical Center, OH, USA.
Abstract
Breast conservation therapy with excision and adjuvant radiation has become an acceptable alternative to mastectomy in treating early-stage breast cancer. This report of a case of severe radiation-induced fibrosis following breast conservation therapy serves to inform the oncologic community of the multimodal approach to non-operative management of radiation-induced fibrosis and indications for surgery in the treatment of this complication arising from external beam radiation.
Introduction
Breast cancer affects approximately 1 in 8 women, and female breast cancer has now surpassed lung cancer as the most diagnosed cancer worldwide [1]. External beam radiation is routinely administered to the breast during breast conservation therapy, and chronic dermatologic side effects from adjuvant radiation therapy can be seen [2,3]. Some of radiation therapy’s more common skin complications include dermatitis, telangiectasias, atrophy, and pain. These effects are often self-limiting and do not require specific treatment. Although rare, significant radiation-induced fibrosis is one of the more difficult to treat side effects of breast irradiation [2].
Given that the overall 10-year survival rate for patients diagnosed with breast cancer is now over 80% and the focus on breast conservation therapy, it is expected that this complication will be seen more frequently in the future [1]. The true incidence of radiation-induced fibrosis is likely on the order of 5%–15% [3–6], although a large proportion of women cite skin changes and discomfort in the irradiated breast. Given the variability in defining and determining the severity of this process, it is likely that the incidence of this complication is under-reported.
Here, we present the case of a patient who developed severe radiation-induced fibrosis of the right breast following breast conservation therapy. The patient had undergone wide local excision, adjuvant radiation, and hormone therapy for stage I breast cancer approximately thirty years prior to presentation. This case report details the presentation, diagnosis, and treatment of radiation fibrosis.
Case Presentation
An 80-year-old Caucasian female patient presented with a chief complaint of skin ulceration and drainage from the upper right breast for the past year. The patient had a past history of stage I right breast cancer treated with breast conservation therapy in 1988. At that time, the patient underwent wide local excision of the primary in conjunction with axillary lymph node sampling, followed by external beam radiation and seven years of adjuvant tamoxifen. The pathology report revealed the tumor to be an “infiltrating duct carcinoma” (likely an invasive ductal carcinoma) that measured 1.9 cm in greatest diameter but gave no further detail on the grade or receptor status. The radiation fields and dosage employed was unknown, but the patient reported skin sloughing and wound drainage for three months following the completion of radiation treatment. Following this, the breast became firm and immobile, and this process progressed over the ensuing years. The patient developed intermittent episodes of presumed right breast cellulitis approximately 20 years after initial diagnosis and breast conservation therapy. There were also episodes of skin breakdown with subsequent serous fluid drainage. These areas healed slowly with conservative measures and local wound care but always recurred within a few months. The patient further experienced moderate breast discomfort and localized pain, which were intermittent at first, but progressively worsened and became more constant as the years passed. At the time of her first consultation with surgical oncology in August 2018, she had been treating an open wound on the right breast for the past six months. The patient’s medical problems included pre-diabetes controlled with diet, atrial fibrillation, hypertension, hypothyroidism, and hypercholesterolemia. The patient denied a history of collagen vascular disease. Her outpatient medications at the time of her visit included losartan, metoprolol, simvastatin, levothyroxine, and warfarin. The patient was a non-smoker, and her family history was significant for breast cancer in her mother at the age of 83.
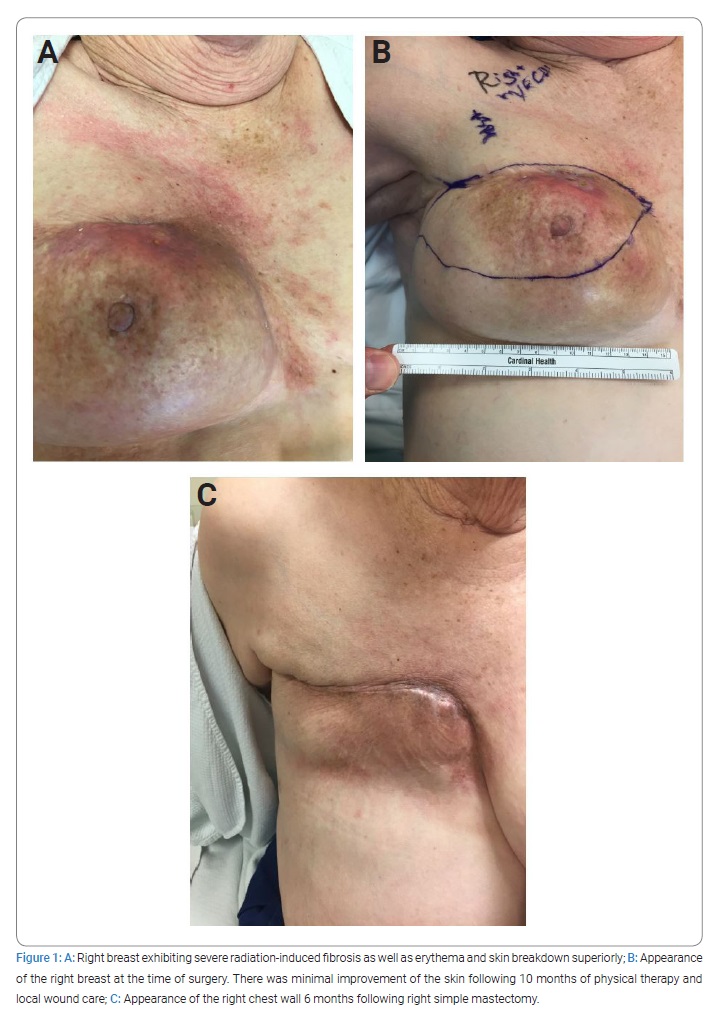
On examination, the right breast was reduced in volume compared to the contralateral side. A transverse scar was noted over the central upper pole of the right breast. It measured 10 cm in length. Further examination revealed pigmentation changes consistent with prior radiation as well as large patches of erythema and skin induration involving the breast and chest wall (Figure 1A, Figure 1B). An open wound was noted approximately 4 cm–6 cm above the nipple, measuring 2 cm in diameter. It was draining clear straw-colored fluid. The underlying breast parenchyma was indurated, and the deep tissues of the medial and lateral aspects of the breast were very firm due to presumed fat necrosis and subsequent deposition of calcium. There was no evidence of pitting edema within the breast tissue.
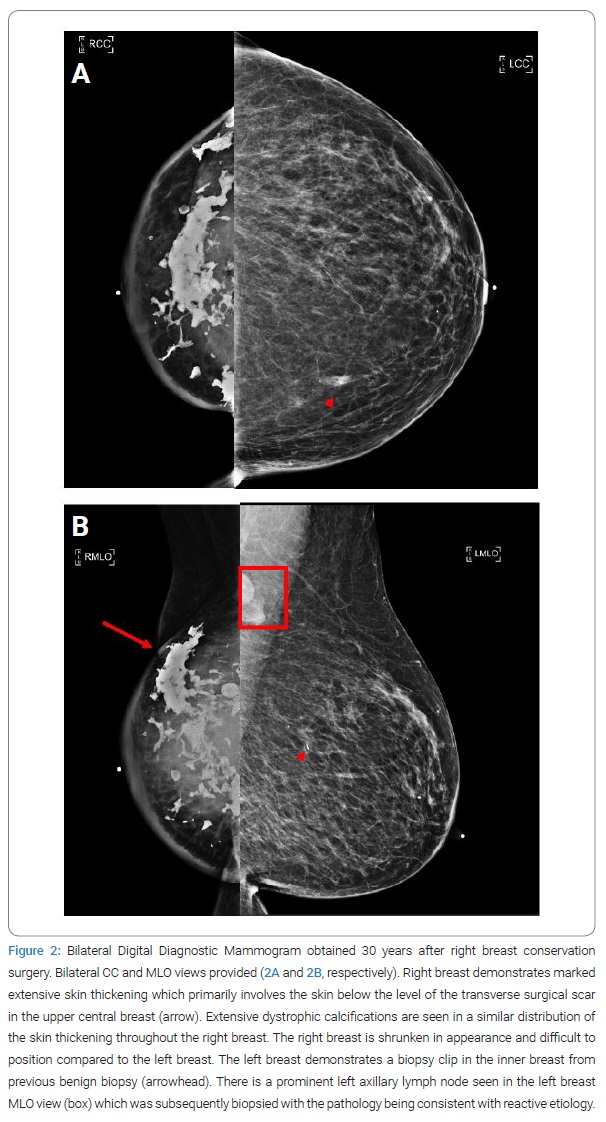
A bilateral diagnostic digital mammogram was obtained and showed dramatic asymmetrical involution of the right breast with marked skin thickening below the level of the surgical scar, corresponding with the clinical examination findings (Figure 2A). Extensive dystrophic calcifications were seen in a similar distribution in the right breast. The left breast demonstrated normal benign-appearing breast parenchyma. A stable biopsy clip was seen in the left medial breast from the previous biopsy, which was reported as a benign lesion. A prominent left axillary lymph node was seen on the mammogram, corresponding to findings on physical examination (Figure 2B). This node was subsequently biopsied under ultrasound guidance, with pathology showing reactive follicular hyperplasia and no evidence of malignancy. A punch biopsy of the right breast’s affected skin was performed simultaneously and revealed dermal fibrosis with chronic inflammation, negative for carcinoma.
A presumptive diagnosis of severe radiation-induced fibrosis of the right breast was made. Treatment with dry dressing changes and applying 1% cortisone cream to the surrounding skin was initiated. The patient was also counseled on using proper breast support and referred to physical therapy for massage therapy. On two subsequent visits approximately two months apart, the patient exhibited no improvement in her physical examination or her symptoms, including pain and drainage from her right breast. At that point, the option of total mastectomy was presented to the patient. After several discussions, the patient agreed to proceed with surgical intervention.
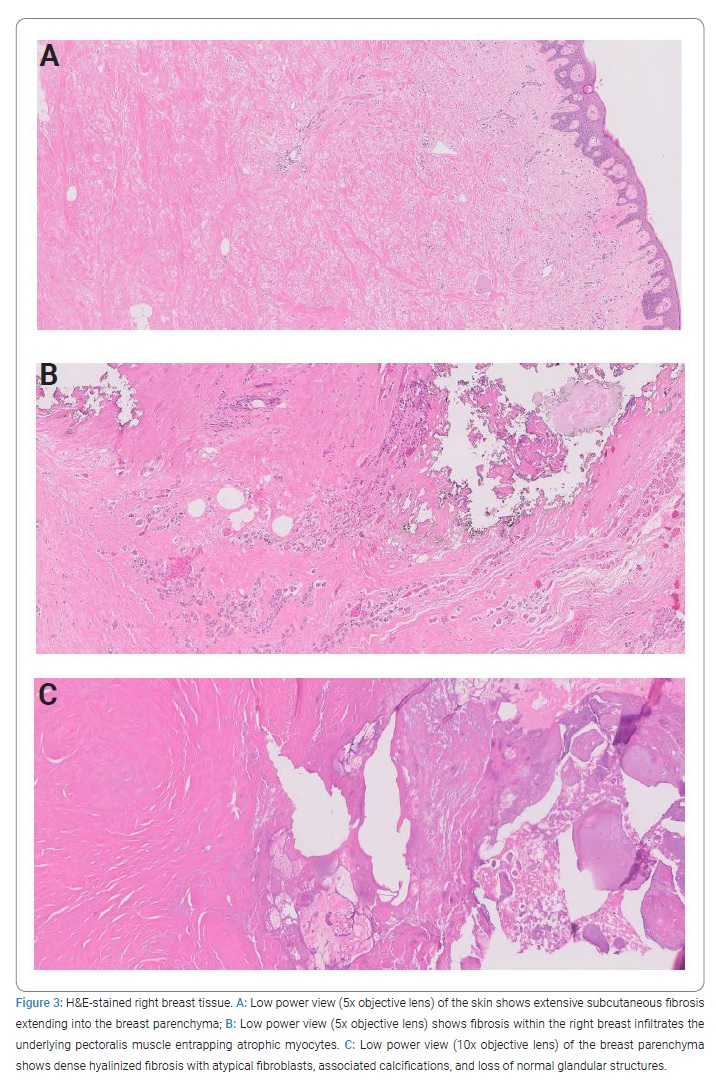
The patient was prepared for surgery, and consultation was made with Plastic Surgery colleagues in the event that an extensive resection of fibrotic skin required a complex skin closure. Cardiac clearance was obtained preoperatively, and the patient was transitioned to therapeutic enoxaparin. After induction of anesthesia, the breast was carefully examined. A surgical plan for a mastectomy included the central breast skin primarily affected by fibrosis. A simple right mastectomy was performed via a transversely oriented Stewart incision incorporating the nipple complex and the horizontal scar in the upper breast. The upper skin flap was developed without difficulty. The lower skin flap was thickened and edematous. As the lateral and medial flaps were created, the presence of significant calcifications in the subcutaneous tissues was noted medially, inferiorly, and laterally. These calcified areas were included within the specimen. There was an area of chalky white material 8 cm in diameter and 1 cm in thickness located in the inner lower quadrant near the sternal border that was adherent to the undersurface of the skin flap. This tissue was removed from the overlying skin using a rongeur and curette. The breast was reflected off the pectoralis major muscle without difficulty. The muscle appeared normal superiorly but was pale and atrophic inferiorly. The lower mastectomy skin flap was adherent to an area of firm scar tissue within the parenchyma. This was carefully resected from the undersurface of the skin flap. Examination of the undersurface of the lateral skin flap revealed additional calcified scar tissue, and this was resected sharply with a ten-blade scalpel. The operation removed the majority of the radiation-affected tissue, and no overt evidence of carcinoma was found. At this point, the skin flaps appeared viable, and the incision was able to be closed over two Jackson-Pratt 19 Fr drains. The patient tolerated the procedure without apparent complications. The drains were removed within two weeks of surgery. The incision healed well, and the patient voiced complete resolution of her symptoms at the 6-month postoperative visit (Figure 1C). The patient had no further episodes of skin breakdown at the one-year postoperative visit. Pathologic examination of the right breast tissue after H & E staining showed extensive fibrosis extending into the breast parenchyma with infiltration of the underlying pectoralis muscle and subsequent entrapment of atrophic myocytes (Figure 3A,Figure 3B). Additionally, dense hyalinized fibrosis was noted with atypical fibroblasts, associated calcifications, and loss of normal glandular structures (Figure 3C). There was no underlying carcinoma or neoplasia identified. The findings of dense fibrosis with associated calcifications and infiltration of the breast parenchyma were felt to be consistent with the clinical diagnosis of radiation-induced fibrosis.
Discussion and Review of the Literature
This report summarizes the case of a patient with severe radiation-induced breast fibrosis after undergoing breast conservation therapy, resulting in an open wound that did not respond to physical therapy or standard wound care measures. After a diagnostic workup to rule out locoregional recurrence and subsequent aggressive medical management, mastectomy was performed for symptom relief. The patient healed well following mastectomy despite the presence of severe skin changes and experienced resolution of the symptoms prompting her initial presentation. Principles of care that might be applied to other patients with this condition include 1) Biopsy of the affected area to rule out the possibility of breast cancer recurrence; 2) Antibiotic therapy for suspected cellulitis; 3) Initiation of physical therapy to include skin massage; 4) Local wound care for areas of skin breakdown; and 5) Patient education regarding expected results. These conservative efforts should be instituted in a consistent manner over a prolonged period of time (at least six months) in order to provide evidence of recalcitrance. Surgical resection of affected tissues should only be considered in cases where there is significant discomfort and/or significant disruption of skin integrity. A consultation with colleagues in Plastic Surgery should be considered in the event that the removal of affected tissues results in a need for assistance in providing adequate skin coverage of the chest wall at the time of resection.
The biological effects of locoregional radiotherapy are well-known, and in severe cases, the changes can evolve into radiation-induced fibrosis. The formation of free radicals and a subsequent inflammatory response leads to increased tumor growth factor beta (TGF-β) levels, driving disorganized collagen deposition and scarring [2]. The primary treatment-related factors are the total dose of radiotherapy (and dose per fraction), the volume of tissue being treated, and the time course over which treatment is delivered [7]. The degree of fibrosis appears to directly correlate with increased radiation dosage, large field size, and prolonged therapy [8,9]. Clinically, radiation-induced fibrosis is characterized by skin retraction and atrophy, as well as firmness to palpation. The skin consequently exhibits functional limitations, and there is a marked decrease in tissue compliance. Histopathology is initially characterized by histiocyte-driven inflammation. With time, the affected area shows evidence of organized fibrosis in which tissues acquire dense fibrotic fields rich in myofibroblasts. As the process evolves and matures, there is progressive fibrosis with loss of cellular components [10]. In some instances, inflamed skin and firm fibrosis can take on the appearance of a local breast cancer recurrence. Unfortunately, detailed case reports are not common in the literature. One report details the case of a breast cancer patient treated with breast-conserving surgery and radiation therapy who presented 12 years later with ipsilateral lymphedema, a right axillary mass, and pain with the arm and shoulder mobilization. Her physical examination and radiologic findings revealed a large mass invading the right thoracic wall and axilla that was concerning for a local recurrence of breast cancer. A pathological evaluation of tissue obtained via open biopsy revealed hyalinized fibrotic tissue with only rare fibroblast-like spindle and lymphoid cells. The final microscopic diagnosis was one of late-term radiation-induced fibrosis with hyaline changes. The patient was treated with anti-inflammatory drugs and tamoxifen, and she was placed on regular follow-up with no further surgical intervention [10].
Physical therapy is an important starting point in treating radiation-induced fibrosis and can significantly improve in some cases. Massage therapy can be quite effective, especially when applied early in the course of the fibrotic process. A device-assisted form of massage was described by Bourgeois and colleagues and may be thought of as a mechanical massage technique that permits skin mobilization by way of folding and unfolding [11]. This group employed the Cellu-M50 LPG Systems medical device (LPG Systems, Valence, France), which mobilizes tissue between two rollers creating a skin fold and stretching the underlying tissue. The treatment was applied to the irradiated breast for 10 minutes three times per week for a total of 5 weeks (15 sessions). Their results were compared to a similar group of patients who did not receive any specific therapy. The LPG treatment group exhibited a decrease in breast erythema, pain, and pruritus and also a subjective decrease in skin induration. Regardless of the type of physical therapy being employed, it is imperative that sufficient time be allotted to the process in order that slow progress is not overlooked. Also, careful attention to wound care can prevent the formation or spread of skin ulcers on the affected breast, and these can be fully healed in time.
Several other interventions have been described for the fibrotic breast post-irradiation, but none have proved overwhelmingly effective. Anti-inflammatory drugs may provide symptomatic relief but have not been reported capable of reversing fibrosis. Although tamoxifen has been prescribed for this condition in case of reports, [11] a review of the literature shows that the administration of tamoxifen during radiotherapy could worsen radiation-induced toxicity when given concurrently with external beam irradiation [12]. However, this concern was not supported by a meta-analysis comparing breast radiotherapy delivered with concurrent or sequential endocrine therapy [13]. Once established, there is no evidence that tamoxifen administration would worsen the condition. Jacobson and colleagues demonstrated improved tissue compliance in breast cancer patients post-irradiation when an anti-inflammatory agent (pentoxifylline) in combination with an antioxidant (vitamin E) was given for six months after irradiation as a means of preventing radiation-induced fibrosis [14]. Pirfenidone modulates transforming growth factor beta (TGF-β) and has been employed for the treatment of idiopathic pulmonary fibrosis and breast implant capsular contraction [15]. Therefore, it has been proposed as a treatment for radiation-induced fibrosis. Teguh et al. employed hyperbaric oxygen treatment (average of 47 sessions) in 57 women with what was termed late radiation-induced toxicity. Pain, breast swelling/sensitivity, and skin irritation were all improved post-therapy [16]. This treatment was well-tolerated but obviously must be administered at a specialized facility. Also, the duration of the results may vary per patient.
In some instances, conservative measures fail, and the patient continues to have significant symptoms. In such cases, surgery might be contemplated as a means to eliminate damaged tissues. Van Geel and colleagues described a series of nine patients who exhibited significant radiation-induced fibrosis that was resistant to conservative measures. They performed a partial mastectomy in order to remove the fibrotic area and followed this with an immediate latissimus dorsi musculocutaneous flap in order to preserve breast shape and volume. The clinical diagnosis of radiation-induced fibrosis was confirmed on pathology, and no surgical complications were reported. All but one patient experienced symptomatic relief, and that patient later went on to have a total mastectomy [17]. To our knowledge, this brief mention of mastectomy for the treatment of radiation-induced fibrosis is the only other time this treatment approach is mentioned in the literature. When the area affected by fibrosis is so extensive that wide local excision is unlikely to remediate the problem, then primary total mastectomy can be considered. A balance must be achieved between resection of the abnormal fibrotic dermis and the retention of skin that shows evidence of effects from radiation but is still able to heal onto the underlying musculature. The photos provided in (Figure 1C) reveal that some of the retained skin exhibited significant radiation damage and yet was able to heal in an adequate fashion. The use of rotational flaps and the latissimus dorsi muscle myocutaneous flap, which Plastic Surgery colleagues can perform, represent accepted methods for providing skin coverage in cases where large amounts of abnormal skin must be resected.
Conclusion
Breast cancer is being diagnosed with increased frequency and at earlier stages with advances in diagnostic imaging and screening practices. Breast conservation therapy with excision, adjuvant radiation, and hormone therapy has become an acceptable and often preferred alternative to simple mastectomy in treating early-stage breast cancer with improved patient satisfaction and cosmetic outcomes. This case of severe radiation-induced fibrosis refractory to maximal medical management after undergoing breast conservation therapy nearly thirty years prior serves to alert the oncologic community to this difficult-to-treat complication of external beam radiation and the role of surgical intervention in the treatment algorithm. As this complication can cause significant pain, discomfort, and disfiguration for the patient, it is important to continue researching preventative methods and non-surgical therapies for radiation fibrosis. In the setting of refractory fibrosis or other complicating factors such as open wounds, recurrent cellulitis, or the inability to rule out locoregional malignant recurrence, it is reasonable to consider surgical excision of the affected tissue, with or without reconstruction by Plastic Surgery colleagues for cosmesis or coverage of large tissue deficits. Hopefully, future efforts will provide new avenues of therapy for preventing severe radiation-induced fibrosis after adjuvant radiation therapy in the context of breast conservation therapy.
Acknowledgments
Grant Support: This work was supported by NIH Grant P30-CA16058.
Author Contribution: Greenblat M/Johnson C: Main authors of the manuscript and primarily conducted the research and writing of this clinical case under the guidance and recommendations of the following co-others and principal investigator; Mangino A: Provided the patient consent information and administrative support; Skoracki R/ Sorkin M: Provided expert consultation in regard to understanding the role of plastic surgery for reconstruction; Mangino A/Tozbikian G: Provided expert consultation and pathologic examination of surgical specimen with preparation of slides; CS: provided expert consultation of radiographic findings; William E. Carson: Contributed to this case as the surgeon and clinician of this patient and developed the original concept for this manuscript including revisions and critical review.
All of the authors were provided manuscript drafts throughout the writing process in order to aid in the development of the finial submission.
Consent: Informed consent was obtained prior to the publication of this case report.
Conflict of Interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Informed consent was obtained for this publication.
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249.
- Yang X, Ren H, Guo C, Hu C, Fu J. Radiation-induced skin injury: pathogenesis, treatment, and management. Aging (Albany NY). 2020;12(22):23379–23393.
- Milam EC, Rangel LK, Pomeranz MK. Dermatologic sequelae of breast cancer: From disease, surgery, and radiation. Int J Dermatol. 2021;60(4):394–406.
- White J, Joiner MC. Toxicity from radiation in breast cancer. Cancer Treat Res. 2006;128:65–109.
- Williams NR, Williams S, Kanapathy M, Naderi N, Vavourakis V, Mosahebi A. Radiation-induced fibrosis in breast cancer: A protocol for an observational cross-sectional pilot study for personalised risk estimation and objective assessment. Int J Surg Protoc. 2019;14:9–13.
- Ramseier JY, Ferreira MN, Leventhal JS. Dermatologic toxicities associated with radiation therapy in women with breast cancer. Int J Womens Dermatol. 2020;6(5):349–356.
- Straub JM, New J, Hamilton CD, Lominska C, Shnayder Y, Thomas SM. Radiation-induced fibrosis: mechanisms and implications for therapy. J Cancer Res Clin Oncol. 2015;141(11):1985–1994.
- Fehlauer F, Tribius S, Höller U, Rades D, Kuhlmey A, Bajrovic A, et al. Long-term radiation sequelae after breast-conserving therapy in women with early-stage breast cancer: an observational study using the LENT-SOMA scoring system. Int J Radiat Oncol Biol Phys. 2003;55(3):651–658.
- Allali S, Kirova Y. Radiodermatitis and fibrosis in the context of breast radiation therapy: a critical review. Cancers (Basel). 2021;13(23):5928.
- Sarsenov D, Aktepe F, Özmen V. Radiation fibrosis syndrome imitating breast cancer recurrence; a case report. J Breast Health. 2017;13(1):40–42.
- Bourgeois JF, Gourgou S, Kramar A, Lagarde JM, Guillot B. A randomized, prospective study using the LPG technique in treating radiation-induced skin fibrosis: clinical and profilometric analysis. Skin Res Technol. 2008;14(1):71–76.
- Cecchini MJ, Yu E, Potvin K, D’souza D, Lock M. Concurrent or sequential hormonal and radiation therapy in breast cancer: a literature review. Cureus. 2015;7(10):e364.
- Li YF, Chang L, Li WH, Xiao MY, Wang Y, He WJ, et al. Radiotherapy concurrent versus sequential with endocrine therapy in breast cancer: A meta-analysis. Breast. 2016;27:93–98.
- Jacobson G, Bhatia S, Smith BJ, Button AM, Bodeker K, Buatti J. Randomized trial of pentoxifylline and vitamin E vs standard follow-up after breast irradiation to prevent breast fibrosis, evaluated by tissue compliance meter. Int J Radiat Oncol Biol Phys. 2013;85(3):604–608.
- Simone NL, Soule BP, Gerber L, Augustine E, Smith S, Altemus RM, et al. Oral pirfenidone in patients with chronic fibrosis resulting from radiotherapy: a pilot study. Radiat Oncol. 2007;2:19.
- Teguh DN, Bol Raap R, Struikmans H, Verhoef C, Koppert LB, Koole A, et al. Hyperbaric oxygen therapy for late radiation-induced tissue toxicity: prospectively patient-reported outcome measures in breast cancer patients. Radiat Oncol. 2016;11(1):130.
- Van Geel AN, Lans TE, Haen R, Wai RTJ, Menke-Pluijmers MBE. Partial mastectomy and m. latissimus dorsi reconstruction for radiation-induced fibrosis after breast-conserving cancer therapy. World J Surg. 2011;35(3):568–572.
Keywords
Radiation; Fibrosis; Mastectomy; Surgery
Cite this article
Greenblat M, Johnson C, Mangino A, Skoracki R, Abdulwaasey M, Tozbikian G, et al. Mastectomy for severe radiation-induced fibrosis following breast conservation therapy. Clin Case Rep J. 2022;3(7):1–8.
Copyright
© 2022 William E. Carson. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-4.0).



